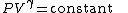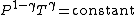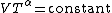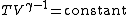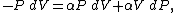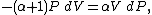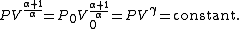
Adiabatic process
Encyclopedia
In thermodynamics
, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat
transfer to or from the working fluid
is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time, so that there is no opportunity for significant heat exchange. The term "adiabatic" literally means impassable, coming from the Greek roots ἀ- ("not"), διὰ- ("through"), and βαῖνειν ("to pass"); this etymology corresponds here to an absence of heat transfer
. Conversely, a process that involves heat transfer (addition or loss of heat to the surroundings) is generally called diabatic. Although the terms adiabatic and isocaloric can often be interchanged, adiabatic processes may be considered a subset of isocaloric processes; the remaining complement subset of isocaloric processes being processes where net heat transfer does not diverge regionally such as in an idealized case with mediums of infinite thermal conductivity or non-existent thermal capacity.
In an adiabatic irreversible process, dQ = 0 is not equal to TdS (TdS > 0). dQ = TdS = 0 holds for reversible processes only.
For example, an adiabatic boundary is a boundary that is impermeable to heat transfer and the system is said to be adiabatically (or thermally) insulated; an insulated wall approximates an adiabatic boundary. Another example is the adiabatic flame temperature
, which is the temperature that would be achieved by a flame
in the absence of heat loss to the surroundings. An adiabatic process that is reversible
is also called an isentropic process
. Additionally, an adiabatic process that is irreversible and extracts no work is in an isenthalpic process, such as viscous drag, progressing towards a nonnegative change in entropy.
One opposite extreme—allowing heat transfer with the surroundings, causing the temperature to remain constant—is known as an isothermal process
. Since temperature is thermodynamically conjugate
to entropy
, the isothermal process is conjugate to the adiabatic process for reversible transformations.
A transformation of a thermodynamic system can be considered adiabatic when it is quick enough that no significant heat is transferred between the system and the outside. At the opposite extreme, a transformation of a thermodynamic system can be considered isothermal if it is slow enough so that the system's temperature remains constant by heat exchange with the outside.
of a gas
while not adding or subtracting any heat
. In contrast, free expansion
is an isothermal process for an ideal gas.
Adiabatic heating occurs when the pressure of a gas is increased from work done on it by its surroundings, e.g. a piston
. Diesel engines rely on adiabatic heating during their compression stroke to elevate the temperature sufficiently to ignite the fuel.
Adiabatic heating also occurs in the Earth's atmosphere
when an air mass
descends, for example, in a katabatic wind
or Foehn wind flowing downhill. When a parcel of air descends, the pressure on the parcel increases. Due to this increase in pressure, the parcel's volume decreases and its temperature increases, thus increasing the internal energy.
Adiabatic cooling occurs when the pressure of a substance is decreased as it does work on its surroundings. Adiabatic cooling does not have to involve a fluid. One technique used to reach very low temperatures (thousandths and even millionths of a degree above absolute zero) is adiabatic demagnetisation, where the change in magnetic field
on a magnetic material is used to provide adiabatic cooling. Adiabatic cooling occurs in the Earth's atmosphere
with orographic lifting and lee waves
, and this can form pileus
or lenticular cloud
s if the air is cooled below the dew point
. Also, the contents of an expanding universe (to first order) can be described as an adiabatically cooling fluid. When the pressure applied on a parcel of air decreases, the air in the parcel is allowed to expand; as the volume increases, the temperature falls and internal energy decreases.
Rising magma also undergoes adiabatic cooling before eruption.
Such temperature changes can be quantified using the ideal gas law
, or the hydrostatic equation for atmospheric processes.
No process is truly adiabatic. Many processes are close to adiabatic and can be easily approximated by using an adiabatic assumption, but there is always some heat loss; as no perfect insulators exist.
 The mathematical equation for an ideal gas
The mathematical equation for an ideal gas
undergoing a reversible (i.e., no entropy generation) adiabatic process is
where P is pressure, V is specific
or molar volume
, and
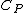 being the specific heat for constant pressure,
being the specific heat for constant pressure,
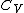 being the specific heat for constant volume, γ is the adiabatic index, and
being the specific heat for constant volume, γ is the adiabatic index, and  is the number of degrees of freedom
is the number of degrees of freedom
divided by 2 (3/2 for monatomic gas, 5/2 for diatomic gas).
For a monatomic ideal gas, , and for a diatomic gas (such as nitrogen
, and for a diatomic gas (such as nitrogen
and oxygen
, the main components of air
) . Note that the above formula is only applicable to classical ideal gases and not Bose–Einstein
. Note that the above formula is only applicable to classical ideal gases and not Bose–Einstein
or Fermi gases
.
For reversible adiabatic processes, it is also true that
where T is an absolute temperature.
This can also be written as
 = 7/5), and that the compression ratio of the engine is 10:1 (that is, the 1000 cc volume of uncompressed gas will compress down to 100 cc when the piston goes from bottom to top). The uncompressed gas is at approximately room temperature and pressure (a warm room temperature of ~27 degC or 300 K, and a pressure of 1 bar ~ 100,000 Pa, or about 14.7 PSI, or typical sea-level atmospheric pressure).
= 7/5), and that the compression ratio of the engine is 10:1 (that is, the 1000 cc volume of uncompressed gas will compress down to 100 cc when the piston goes from bottom to top). The uncompressed gas is at approximately room temperature and pressure (a warm room temperature of ~27 degC or 300 K, and a pressure of 1 bar ~ 100,000 Pa, or about 14.7 PSI, or typical sea-level atmospheric pressure).

so our adiabatic constant for this experiment is about 1.58 billion.
The gas is now compressed to a 100cc volume (we will assume this happens quickly enough that no heat can enter or leave the gas). The new volume is 100 ccs, but the constant for this experiment is still 1.58 billion:
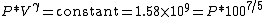
so solving for P:

or about 362 PSI or 24.5 atm. Note that this pressure increase is more than a simple 10:1 compression ratio would indicate; this is because the gas is not only compressed, but the work done to compress the gas has also heated the gas and the hotter gas will have a greater pressure even if the volume had not changed.
We can solve for the temperature of the compressed gas in the engine cylinder as well, using the ideal gas law.
Our initial conditions are 100,000 pa of pressure, 1000 cc volume, and 300 K of temperature, so our experimental constant is:

We know the compressed gas has V = 100 cc and P = 2.50E6 pascals, so we can solve for temperature by simple algebra:

That's 751 Kelvins, or 477 °C, or 892 °F. This is why a high compression engine requires fuels specially formulated to not self-ignite (which would cause engine knocking
when operated under these conditions of temperature and pressure), or that a supercharger
and intercooler
to provide a lower temperature at the same pressure would be advantageous. A diesel engine
operates under even more extreme conditions, with compression ratios of 20:1 or more being typical, in order to provide a very high gas temperature which insures immediate ignition of injected fuel.
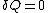 . Then, according to the first law of thermodynamics
. Then, according to the first law of thermodynamics
,
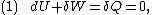
where dU is the change in the internal energy of the system and δW is work done
by the system. Any work (δW) done must be done at the expense of internal energy U, since no heat δQ is being supplied from the surroundings. Pressure-volume work δW done by the system is defined as

However, P does not remain constant during an adiabatic process but
instead changes along with V.
It is desired to know how the values of dP and
dV relate to each other as the adiabatic process proceeds.
For an ideal gas the internal energy is given by

where is the number of degrees of freedom
is the number of degrees of freedom
divided by two, R is the universal gas constant and n is the number of moles in the system (a constant).
Differentiating Equation (3) and use of the ideal gas law
, , yields
, yields

Equation (4) is often expressed as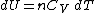
because .
.
Now substitute equations (2) and (4) into equation (1) to obtain
simplify:
and divide both sides by PV:
After integrating the left and right sides from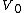 to V and from
to V and from  to P and changing the sides respectively,
to P and changing the sides respectively,
Exponentiate both sides,
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
transfer to or from the working fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time, so that there is no opportunity for significant heat exchange. The term "adiabatic" literally means impassable, coming from the Greek roots ἀ- ("not"), διὰ- ("through"), and βαῖνειν ("to pass"); this etymology corresponds here to an absence of heat transfer
Heat transfer
Heat transfer is a discipline of thermal engineering that concerns the exchange of thermal energy from one physical system to another. Heat transfer is classified into various mechanisms, such as heat conduction, convection, thermal radiation, and phase-change transfer...
. Conversely, a process that involves heat transfer (addition or loss of heat to the surroundings) is generally called diabatic. Although the terms adiabatic and isocaloric can often be interchanged, adiabatic processes may be considered a subset of isocaloric processes; the remaining complement subset of isocaloric processes being processes where net heat transfer does not diverge regionally such as in an idealized case with mediums of infinite thermal conductivity or non-existent thermal capacity.
In an adiabatic irreversible process, dQ = 0 is not equal to TdS (TdS > 0). dQ = TdS = 0 holds for reversible processes only.
For example, an adiabatic boundary is a boundary that is impermeable to heat transfer and the system is said to be adiabatically (or thermally) insulated; an insulated wall approximates an adiabatic boundary. Another example is the adiabatic flame temperature
Adiabatic flame temperature
In the study of combustion, there are two types of adiabatic flame temperature depending on how the process is completed, constant volume and constant pressure, describing the temperature the combustion products theoretically reach if no energy is lost to the outside environment.The constant volume...
, which is the temperature that would be achieved by a flame
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
in the absence of heat loss to the surroundings. An adiabatic process that is reversible
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
is also called an isentropic process
Isentropic process
In thermodynamics, an isentropic process or isoentropic process is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the...
. Additionally, an adiabatic process that is irreversible and extracts no work is in an isenthalpic process, such as viscous drag, progressing towards a nonnegative change in entropy.
One opposite extreme—allowing heat transfer with the surroundings, causing the temperature to remain constant—is known as an isothermal process
Isothermal process
An isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
. Since temperature is thermodynamically conjugate
Conjugate variables (thermodynamics)
In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature/entropy or pressure/volume. In fact all thermodynamic potentials are expressed in terms of conjugate pairs....
to entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, the isothermal process is conjugate to the adiabatic process for reversible transformations.
A transformation of a thermodynamic system can be considered adiabatic when it is quick enough that no significant heat is transferred between the system and the outside. At the opposite extreme, a transformation of a thermodynamic system can be considered isothermal if it is slow enough so that the system's temperature remains constant by heat exchange with the outside.
Adiabatic heating and cooling
Adiabatic changes in temperature occur due to changes in pressurePressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
of a gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
while not adding or subtracting any heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
. In contrast, free expansion
Free expansion
Free expansion is an irreversible process in which a gas expands into an insulated evacuated chamber.Real gases experience a temperature change during free expansion...
is an isothermal process for an ideal gas.
Adiabatic heating occurs when the pressure of a gas is increased from work done on it by its surroundings, e.g. a piston
Piston
A piston is a component of reciprocating engines, reciprocating pumps, gas compressors and pneumatic cylinders, among other similar mechanisms. It is the moving component that is contained by a cylinder and is made gas-tight by piston rings. In an engine, its purpose is to transfer force from...
. Diesel engines rely on adiabatic heating during their compression stroke to elevate the temperature sufficiently to ignite the fuel.
Adiabatic heating also occurs in the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
when an air mass
Air mass
In meteorology, an air mass is a volume of air defined by its temperature and water vapor content. Air masses cover many hundreds or thousands of square miles, and adopt the characteristics of the surface below them. They are classified according to latitude and their continental or maritime...
descends, for example, in a katabatic wind
Katabatic wind
A katabatic wind, from the Greek word katabatikos meaning "going downhill", is the technical name for a drainage wind, a wind that carries high density air from a higher elevation down a slope under the force of gravity. Such winds are sometimes also called fall winds...
or Foehn wind flowing downhill. When a parcel of air descends, the pressure on the parcel increases. Due to this increase in pressure, the parcel's volume decreases and its temperature increases, thus increasing the internal energy.
Adiabatic cooling occurs when the pressure of a substance is decreased as it does work on its surroundings. Adiabatic cooling does not have to involve a fluid. One technique used to reach very low temperatures (thousandths and even millionths of a degree above absolute zero) is adiabatic demagnetisation, where the change in magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
on a magnetic material is used to provide adiabatic cooling. Adiabatic cooling occurs in the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
with orographic lifting and lee waves
Lee waves
In meteorology, lee waves are atmospheric standing waves. The most common form is mountain waves, which are atmospheric internal gravity waves...
, and this can form pileus
Pileus (meteorology)
A pileus , also called scarf cloud or cap cloud, is a small, horizontal cloud that can appear above a cumulus or cumulonimbus cloud, giving the parent cloud a characteristic "hoodlike" appearance. Pilei tend to change shape rapidly. They are formed by strong updrafts acting upon moist air at lower...
or lenticular cloud
Lenticular cloud
Lenticular clouds are stationary lens-shaped clouds that form at high altitudes, normally aligned perpendicular to the wind direction. Lenticular clouds can be separated into altocumulus standing lenticularis , stratocumulus standing lenticular , and cirrocumulus standing lenticular...
s if the air is cooled below the dew point
Dew point
The dew point is the temperature to which a given parcel of humid air must be cooled, at constant barometric pressure, for water vapor to condense into liquid water. The condensed water is called dew when it forms on a solid surface. The dew point is a saturation temperature.The dew point is...
. Also, the contents of an expanding universe (to first order) can be described as an adiabatically cooling fluid. When the pressure applied on a parcel of air decreases, the air in the parcel is allowed to expand; as the volume increases, the temperature falls and internal energy decreases.
Rising magma also undergoes adiabatic cooling before eruption.
Such temperature changes can be quantified using the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
, or the hydrostatic equation for atmospheric processes.
No process is truly adiabatic. Many processes are close to adiabatic and can be easily approximated by using an adiabatic assumption, but there is always some heat loss; as no perfect insulators exist.
Ideal gas (reversible process)

Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
undergoing a reversible (i.e., no entropy generation) adiabatic process is
where P is pressure, V is specific
Specific volume
In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass. It is the reciprocal of density:In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass...
or molar volume
Molar volume
The molar volume, symbol Vm, is the volume occupied by one mole of a substance at a given temperature and pressure. It is equal to the molar mass divided by the mass density...
, and
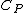 being the specific heat for constant pressure,
being the specific heat for constant pressure,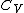 being the specific heat for constant volume, γ is the adiabatic index, and
being the specific heat for constant volume, γ is the adiabatic index, and  is the number of degrees of freedom
is the number of degrees of freedomDegrees of freedom (physics and chemistry)
A degree of freedom is an independent physical parameter, often called a dimension, in the formal description of the state of a physical system...
divided by 2 (3/2 for monatomic gas, 5/2 for diatomic gas).
For a monatomic ideal gas,
 , and for a diatomic gas (such as nitrogen
, and for a diatomic gas (such as nitrogenNitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, the main components of air
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
)
 . Note that the above formula is only applicable to classical ideal gases and not Bose–Einstein
. Note that the above formula is only applicable to classical ideal gases and not Bose–EinsteinBose–Einstein condensate
A Bose–Einstein condensate is a state of matter of a dilute gas of weakly interacting bosons confined in an external potential and cooled to temperatures very near absolute zero . Under such conditions, a large fraction of the bosons occupy the lowest quantum state of the external potential, at...
or Fermi gases
Fermionic condensate
A fermionic condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate, a superfluid phase formed by bosonic atoms under similar conditions. Unlike the Bose–Einstein condensates, fermionic condensates are formed using...
.
For reversible adiabatic processes, it is also true that
where T is an absolute temperature.
This can also be written as
Example of adiabatic compression
Let's now look at a common example of adiabatic compression- the compression stroke in a gasoline engine. We will make a few simplifying assumptions: that the uncompressed volume of the cylinder is 1000cc's (one liter), that the gas within is nearly pure nitrogen (thus a diatomic gas with five degrees of freedom and so = 7/5), and that the compression ratio of the engine is 10:1 (that is, the 1000 cc volume of uncompressed gas will compress down to 100 cc when the piston goes from bottom to top). The uncompressed gas is at approximately room temperature and pressure (a warm room temperature of ~27 degC or 300 K, and a pressure of 1 bar ~ 100,000 Pa, or about 14.7 PSI, or typical sea-level atmospheric pressure).
= 7/5), and that the compression ratio of the engine is 10:1 (that is, the 1000 cc volume of uncompressed gas will compress down to 100 cc when the piston goes from bottom to top). The uncompressed gas is at approximately room temperature and pressure (a warm room temperature of ~27 degC or 300 K, and a pressure of 1 bar ~ 100,000 Pa, or about 14.7 PSI, or typical sea-level atmospheric pressure).
so our adiabatic constant for this experiment is about 1.58 billion.
The gas is now compressed to a 100cc volume (we will assume this happens quickly enough that no heat can enter or leave the gas). The new volume is 100 ccs, but the constant for this experiment is still 1.58 billion:
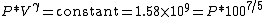
so solving for P:

or about 362 PSI or 24.5 atm. Note that this pressure increase is more than a simple 10:1 compression ratio would indicate; this is because the gas is not only compressed, but the work done to compress the gas has also heated the gas and the hotter gas will have a greater pressure even if the volume had not changed.
We can solve for the temperature of the compressed gas in the engine cylinder as well, using the ideal gas law.
Our initial conditions are 100,000 pa of pressure, 1000 cc volume, and 300 K of temperature, so our experimental constant is:

We know the compressed gas has V = 100 cc and P = 2.50E6 pascals, so we can solve for temperature by simple algebra:

That's 751 Kelvins, or 477 °C, or 892 °F. This is why a high compression engine requires fuels specially formulated to not self-ignite (which would cause engine knocking
Engine knocking
Knocking in spark-ignition internal combustion engines occurs when combustion of the air/fuel mixture in the cylinder starts off correctly in response to ignition by the spark plug, but one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front.The...
when operated under these conditions of temperature and pressure), or that a supercharger
Supercharger
A supercharger is an air compressor used for forced induction of an internal combustion engine.The greater mass flow-rate provides more oxygen to support combustion than would be available in a naturally aspirated engine, which allows more fuel to be burned and more work to be done per cycle,...
and intercooler
Intercooler
An intercooler , or charge air cooler, is an air-to-air or air-to-liquid heat exchange device used on turbocharged and supercharged internal combustion engines to improve their volumetric efficiency by increasing intake air charge density through nearly isobaric cooling, which removes...
to provide a lower temperature at the same pressure would be advantageous. A diesel engine
Diesel engine
A diesel engine is an internal combustion engine that uses the heat of compression to initiate ignition to burn the fuel, which is injected into the combustion chamber...
operates under even more extreme conditions, with compression ratios of 20:1 or more being typical, in order to provide a very high gas temperature which insures immediate ignition of injected fuel.
Adiabatic free expansion of a gas
For an adiabatic free expansion process, the gas is contained in an insulated container and a vacuum. The gas is then allowed to expand in the vacuum. The work done by or on the system is zero, because the volume of the container does not change. The First Law of Thermodynamics then implies that the net internal energy change of the system is zero. For an ideal gas, the temperature remains constant because the internal energy only depends on temperature in that case. Since at constant temperature, the entropy is proportional to the volume, the entropy increases in this case, therefore this process is irreversible.Derivation of continuous formula for adiabatic heating and cooling
The definition of an adiabatic process is that heat transfer to the system is zero,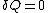 . Then, according to the first law of thermodynamics
. Then, according to the first law of thermodynamicsFirst law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
,
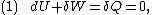
where dU is the change in the internal energy of the system and δW is work done
by the system. Any work (δW) done must be done at the expense of internal energy U, since no heat δQ is being supplied from the surroundings. Pressure-volume work δW done by the system is defined as

However, P does not remain constant during an adiabatic process but
instead changes along with V.
It is desired to know how the values of dP and
dV relate to each other as the adiabatic process proceeds.
For an ideal gas the internal energy is given by

where
 is the number of degrees of freedom
is the number of degrees of freedomDegrees of freedom (physics and chemistry)
A degree of freedom is an independent physical parameter, often called a dimension, in the formal description of the state of a physical system...
divided by two, R is the universal gas constant and n is the number of moles in the system (a constant).
Differentiating Equation (3) and use of the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
,
 , yields
, yields
Equation (4) is often expressed as
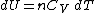
because
 .
.Now substitute equations (2) and (4) into equation (1) to obtain
simplify:
and divide both sides by PV:
After integrating the left and right sides from
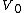 to V and from
to V and from  to P and changing the sides respectively,
to P and changing the sides respectively,Exponentiate both sides,
-
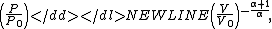
and eliminate the negative sign to obtain
-

Therefore,
-

and
Derivation of discrete formula
The change in internal energy of a system, measured from state 1 to state 2, is equal to

At the same time, the work done by the pressure-volume changes as a result from this process, is equal to
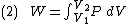
Since we require the process to be adiabatic, the following equation needs to be true
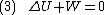
By the previous derivation,
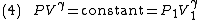
Rearranging (4) gives
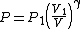
Substituting this into (2) gives
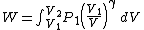
Integrating,
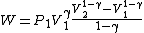
Substituting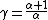 ,
,

Rearranging,

Using the ideal gas law and assuming a constant molar quantity (as often happens in practical cases),
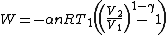
By the continuous formula,

Or,

Substituting into the previous expression for ,
,

Substituting this expression and (1) in (3) gives
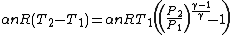
Simplifying,
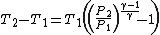
Simplifying,

Simplifying,

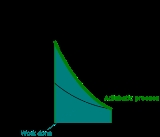
Graphing adiabats
An adiabat is a curve of constant entropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
on the P-V diagram. Properties of adiabats on a P-V diagram are:- Every adiabat asymptotically approachesAsymptoteIn analytic geometry, an asymptote of a curve is a line such that the distance between the curve and the line approaches zero as they tend to infinity. Some sources include the requirement that the curve may not cross the line infinitely often, but this is unusual for modern authors...
both the V axis and the P axis (just like isotherms). - Each adiabat intersects each isotherm exactly once.
- An adiabat looks similar to an isotherm, except that during an expansion, an adiabat loses more pressure than an isotherm, so it has a steeper inclination (more vertical).
- If isotherms are concave towards the "north-east" direction (45 °), then adiabats are concave towards the "east north-east" (31 °).
- If adiabats and isotherms are graphed severally at regular changes of entropy and temperature, respectively (like altitude on a contour map), then as the eye moves towards the axes (towards the south-west), it sees the density of isotherms stay constant, but it sees the density of adiabats grow. The exception is very near absolute zero, where the density of adiabats drops sharply and they become rare (see Nernst's theorem).
The following diagram is a P-V diagram with a superposition of adiabats and isotherms:
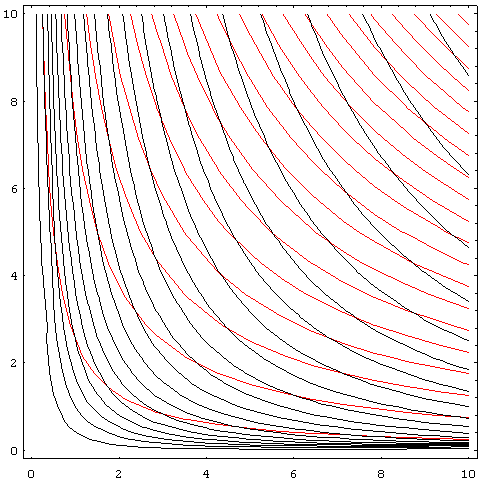
The isotherms are the red curves and the adiabats are the black curves.
The adiabats are isentropic.
Volume is the horizontal axis and pressure is the vertical axis.
See also
- Cyclic process
- First law of thermodynamicsFirst law of thermodynamicsThe first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
- Heat burstHeat burstIn meteorology, a heat burst is a rare atmospheric phenomenon characterised by gusty winds and a rapid increase in temperature and decrease in dew point...
- Isobaric processIsobaric processAn isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,...
- Isenthalpic processIsenthalpic processAn isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, H; or specific enthalpy, h....
- Isentropic processIsentropic processIn thermodynamics, an isentropic process or isoentropic process is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the...
- Isochoric processIsochoric processAn isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant...
- Isothermal processIsothermal processAn isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
- Polytropic process
- Entropy (classical thermodynamics)
- Quasistatic equilibriumQuasistatic equilibriumQuasistatic equilibrium is the quasi-balanced state of a thermodynamic system near to thermodynamic equilibrium in some sense or degree...
- Total air temperatureTotal air temperatureTotal air temperature is a term used generally in aviation. In other applications it is called stagnation temperature. Total air temperature is measured by a specially designed temperature probe mounted on the surface of the aircraft. The probe is designed to bring the air to rest relative to the...
- Adiabatic engine
- Magnetic refrigerationMagnetic refrigerationMagnetic refrigeration is a cooling technology based on the magnetocaloric effect. This technique can be used to attain extremely low temperatures , as well as the ranges used in common refrigerators, depending on the design of the system.The effect was first observed by the German physicist Emil...
External links
-
-
-