
Thermal energy
Encyclopedia
Thermal energy is the part of the total internal energy
of a thermodynamic system
or sample of matter that results in the system's temperature.
The internal energy, also often called the thermodynamic energy, includes other forms of energy in a thermodynamic system in addition to thermal energy, namely forms of potential energy
, such as the chemical energy
stored in its molecular structure and electronic configuration, intermolecular interactions, and the nuclear energy
that binds the sub-atomic particles of matter. It is a free type of energy.
Microscopically, the thermal energy is the kinetic energy
of a system's constituent particles, which may be atoms, molecules, electrons, or particles in plasmas. It originates from the individually random, or disordered, motion of particles in a large ensemble. The thermal energy is equally partitioned
between all available quadratic degrees of freedom
of the particles. These degrees of freedom may include pure translational motion in fluid
s, normal mode
s of vibrations, such as intermolecular vibrations or crystal lattice vibrations, or rotational states. In general, the availability of any such degrees of freedom is a function of the energy in the system, and therefore depends on the temperature.
When two thermodynamic systems with different temperatures are brought into diathermic contact, they exchange energy in form of heat
, which is a transfer of thermal energy from the system of higher temperature to the colder system. This heat may cause work to be performed on each system, for example, in form of volume or pressure changes. This work may be used in heat engines to convert thermal energy into mechanical energy. When two systems have reached a thermodynamic equilibrium
, they have attained the same temperature and the net exchange of thermal energy ceases.
Thermal energy is distinct from heat. In the strict use in physics, heat is a characteristic only of a process, i.e., it is absorbed or produced as an energy exchange, but it is not a static property of matter. Matter does not contain heat, but thermal energy. Heat is thermal energy in the process of transfer or conversion across a boundary of one region of matter to another.
All kinetic energy is partitioned into the degrees of freedom of the system. The average energy of a single particle with f quadratic degrees of freedom in a thermal bath of temperature T is a statistical mean energy given by the equipartition theorem
as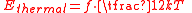
where k is the Boltzmann constant. The total thermal energy of a sample of matter or a thermodynamic system is consequently the average sum of the kinetic energies of all particles in the system. Thus, for a system of N particles its thermal energy is
In general, however, Uthermal is not the total energy of a system. Physical systems also contains static potential energy
(such as chemical energy
) that arises from interactions between particles, nuclear energy associated with atomic nuclei of particles, and even the rest mass energy due to the equivalence of energy and mass.
characterized the terms latent heat
and sensible heat
as components of heat each effecting distinct physical phenomena, namely the potential and kinetic energy of particles, respectively. He describes latent energy as the energy of interaction in a given configuration of particles, i.e., a form of potential energy
, and the sensible heat as an energy affecting the thermal energy, which he called the living force.
, which is well approximated by3 monatomic gas at low pressure. The ideal gas is a gas of particles considered as point objects of perfect spherical symmetry that interact only by elastic collisions and fill a volume such that their mean free path between collisions is much larger than their diameter.
The mechanical kinetic energy of a single particle is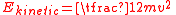
where m is the particle's mass and v is its velocity. The thermal energy of the gas sample consisting of N atoms is given by the sum of these energies, assuming no losses to the container or the environment: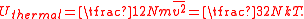
where the line over the velocity term indicates that the average value is calculated over the entire ensemble. The total thermal energy of the sample is proportional to the macroscopic temperature by a constant factor accounting for the three translational degrees of freedom of each particle and the Boltzmann constant, converting units between the microscopic model and the macroscopic temperature. This formalism is the basic assumption that directly yields the ideal gas law, and it shows that for the ideal gas, the internal energy consists only of its thermal energy: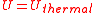
In thermodynamics, heat
must always be defined as energy in exchange between two systems, or a single system and its surroundings. According to the zeroth law of thermodynamics
, heat is exchanged between thermodynamic systems in thermal contact only if their temperatures are different. For the purpose of distinction, a system is defined to be enclosed by a well-characterized boundary. If heat traverses the boundary in direction into the system, the internal energy change is considered to be a positive quantity, while exiting the system, it is negative. Heat is never a property of the system, nor is it contained within the boundary of the system.
In contrast to heat, thermal energy exists on both sides of a boundary. It is the statistical mean of the microscopic fluctuations of the kinetic energy of the systems' particles, and it is the source and the effect of the transfer of heat across a system boundary. Statistically, thermal energy is always exchanged between systems, even when the temperatures on both sides is the same, i.e. the systems are in thermal equilibrium. However, at equilibrium, the net exchange of thermal energy is zero, and therefore there is no heat.
Thermal energy may be increased in a system by other means than heat, for example when mechanical or electrical work is performed on the system. No qualitative difference exists between the thermal energy added by other means. There is also no need in classical thermodynamics to characterize the thermal energy in terms of atomic or molecular behavior. A change in thermal energy induced in a system is the product of the change in entropy and the temperature of the system.
Heat exchanged with a system may cause changes other than a change in thermal energy. For example, it may cause phase transitions, such as melting or evaporation, which are changes in the configuration of a material. Since such an energy exchange is not observable by a change in temperature, it is called a latent heat
and represents a change in the potential energy of the system.
Rather than being itself the thermal energy involved in a transfer, heat is sometimes also understood as the process of transfer, i.e., it functions as a verb.
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
or sample of matter that results in the system's temperature.
The internal energy, also often called the thermodynamic energy, includes other forms of energy in a thermodynamic system in addition to thermal energy, namely forms of potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
, such as the chemical energy
Chemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
stored in its molecular structure and electronic configuration, intermolecular interactions, and the nuclear energy
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
that binds the sub-atomic particles of matter. It is a free type of energy.
Microscopically, the thermal energy is the kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
of a system's constituent particles, which may be atoms, molecules, electrons, or particles in plasmas. It originates from the individually random, or disordered, motion of particles in a large ensemble. The thermal energy is equally partitioned
Equipartition theorem
In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition...
between all available quadratic degrees of freedom
Degrees of freedom (physics and chemistry)
A degree of freedom is an independent physical parameter, often called a dimension, in the formal description of the state of a physical system...
of the particles. These degrees of freedom may include pure translational motion in fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
s, normal mode
Normal mode
A normal mode of an oscillating system is a pattern of motion in which all parts of the system move sinusoidally with the same frequency and with a fixed phase relation. The frequencies of the normal modes of a system are known as its natural frequencies or resonant frequencies...
s of vibrations, such as intermolecular vibrations or crystal lattice vibrations, or rotational states. In general, the availability of any such degrees of freedom is a function of the energy in the system, and therefore depends on the temperature.
When two thermodynamic systems with different temperatures are brought into diathermic contact, they exchange energy in form of heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, which is a transfer of thermal energy from the system of higher temperature to the colder system. This heat may cause work to be performed on each system, for example, in form of volume or pressure changes. This work may be used in heat engines to convert thermal energy into mechanical energy. When two systems have reached a thermodynamic equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
, they have attained the same temperature and the net exchange of thermal energy ceases.
Thermal energy is distinct from heat. In the strict use in physics, heat is a characteristic only of a process, i.e., it is absorbed or produced as an energy exchange, but it is not a static property of matter. Matter does not contain heat, but thermal energy. Heat is thermal energy in the process of transfer or conversion across a boundary of one region of matter to another.
Definitions
Thermal energy is the portion of the internal energy that is responsible for a system's temperature. Microscopically, the thermal energy is identified with mechanical kinetic energy of the constituent particles or other forms of kinetic energy associated with quantum-mechanical microstates. The distinguishing difference between the terms kinetic energy and thermal energy is that thermal energy is the mean energy of disordered, i.e., random, motion of the particles or the oscillations in the system. The conversion of energy of ordered motion to thermal energy results from collisions.All kinetic energy is partitioned into the degrees of freedom of the system. The average energy of a single particle with f quadratic degrees of freedom in a thermal bath of temperature T is a statistical mean energy given by the equipartition theorem
Equipartition theorem
In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition...
as
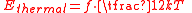
where k is the Boltzmann constant. The total thermal energy of a sample of matter or a thermodynamic system is consequently the average sum of the kinetic energies of all particles in the system. Thus, for a system of N particles its thermal energy is

In general, however, Uthermal is not the total energy of a system. Physical systems also contains static potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
(such as chemical energy
Chemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
) that arises from interactions between particles, nuclear energy associated with atomic nuclei of particles, and even the rest mass energy due to the equivalence of energy and mass.
Historical context
In a 1847 lecture entitled On Matter, Living Force, and Heat, James Prescott JouleJames Prescott Joule
James Prescott Joule FRS was an English physicist and brewer, born in Salford, Lancashire. Joule studied the nature of heat, and discovered its relationship to mechanical work . This led to the theory of conservation of energy, which led to the development of the first law of thermodynamics. The...
characterized the terms latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
and sensible heat
Sensible heat
Sensible heat is the energy exchanged by a thermodynamic system that has as its sole effect a change of temperature.The term is used in contrast to a latent heat, which is the amount of energy exchanged that is hidden, meaning it cannot be observed as a change of temperature...
as components of heat each effecting distinct physical phenomena, namely the potential and kinetic energy of particles, respectively. He describes latent energy as the energy of interaction in a given configuration of particles, i.e., a form of potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
, and the sensible heat as an energy affecting the thermal energy, which he called the living force.
Thermal energy of the ideal gas
Thermal energy is most easily defined in the context of the ideal gasIdeal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
, which is well approximated by3 monatomic gas at low pressure. The ideal gas is a gas of particles considered as point objects of perfect spherical symmetry that interact only by elastic collisions and fill a volume such that their mean free path between collisions is much larger than their diameter.
The mechanical kinetic energy of a single particle is
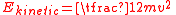
where m is the particle's mass and v is its velocity. The thermal energy of the gas sample consisting of N atoms is given by the sum of these energies, assuming no losses to the container or the environment:
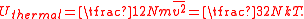
where the line over the velocity term indicates that the average value is calculated over the entire ensemble. The total thermal energy of the sample is proportional to the macroscopic temperature by a constant factor accounting for the three translational degrees of freedom of each particle and the Boltzmann constant, converting units between the microscopic model and the macroscopic temperature. This formalism is the basic assumption that directly yields the ideal gas law, and it shows that for the ideal gas, the internal energy consists only of its thermal energy:
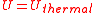
Distinction of thermal energy and heat
In engineering and technology, and particularly in fields that deal with civil energy use and conservation in building construction, heating systems, and power generation, heat and thermal energy are often indiscriminately used interchangeably.In thermodynamics, heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
must always be defined as energy in exchange between two systems, or a single system and its surroundings. According to the zeroth law of thermodynamics
Zeroth law of thermodynamics
The zeroth law of thermodynamics is a generalization principle of thermal equilibrium among bodies, or thermodynamic systems, in contact.The zeroth law states that if two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.Systems are said to...
, heat is exchanged between thermodynamic systems in thermal contact only if their temperatures are different. For the purpose of distinction, a system is defined to be enclosed by a well-characterized boundary. If heat traverses the boundary in direction into the system, the internal energy change is considered to be a positive quantity, while exiting the system, it is negative. Heat is never a property of the system, nor is it contained within the boundary of the system.
In contrast to heat, thermal energy exists on both sides of a boundary. It is the statistical mean of the microscopic fluctuations of the kinetic energy of the systems' particles, and it is the source and the effect of the transfer of heat across a system boundary. Statistically, thermal energy is always exchanged between systems, even when the temperatures on both sides is the same, i.e. the systems are in thermal equilibrium. However, at equilibrium, the net exchange of thermal energy is zero, and therefore there is no heat.
Thermal energy may be increased in a system by other means than heat, for example when mechanical or electrical work is performed on the system. No qualitative difference exists between the thermal energy added by other means. There is also no need in classical thermodynamics to characterize the thermal energy in terms of atomic or molecular behavior. A change in thermal energy induced in a system is the product of the change in entropy and the temperature of the system.
Heat exchanged with a system may cause changes other than a change in thermal energy. For example, it may cause phase transitions, such as melting or evaporation, which are changes in the configuration of a material. Since such an energy exchange is not observable by a change in temperature, it is called a latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
and represents a change in the potential energy of the system.
Rather than being itself the thermal energy involved in a transfer, heat is sometimes also understood as the process of transfer, i.e., it functions as a verb.
Thermal energy of individual particles
The term thermal energy is also often used as a property of single particles to designate the kinetic energy of the particles. An example is the description of thermal neutrons having a certain thermal energy, which means that the kinetic energy of the particle is equivalent to the temperature of its surroundings.See also
- Geothermal energy
- Heat transferHeat transferHeat transfer is a discipline of thermal engineering that concerns the exchange of thermal energy from one physical system to another. Heat transfer is classified into various mechanisms, such as heat conduction, convection, thermal radiation, and phase-change transfer...
- Ocean thermal energy conversionOcean thermal energy conversionOcean Thermal Energy Conversion uses the difference between cooler deep and warmer shallow or surface ocean waters to run a heat engine and produce useful work, usually in the form of electricity....
- Thermal scienceThermal scienceThermal science is the combined study of thermodynamics, fluid mechanics, heat transfer, and combustion.-Overview:Introductory subjects studied in thermal science generally are focused on thermodynamics...

