Organofluorine chemistry
Encyclopedia
Carbon–fluorine bond
The carbon–fluorine bond is a bond between carbon and fluorine that is a component of all organofluorine compounds. It is the strongest single bond in organic chemistry—and relatively short—due to its partial ionic character. The bond also strengthens and shortens as more fluorines are...
. Organofluorine compounds find diverse applications ranging from oil-
Lipophobicity
Lipophobicity, also sometimes called lipophobia, is a chemical property of chemical compounds which means "fat rejection", literally "fear of fat". Lipophobic compounds are those not soluble in lipids or other non-polar solvents...
and water-repellents
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
to pharmaceuticals, refrigerants and reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s in catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. In addition to these applications, some organofluorine compounds are pollutant
Pollutant
A pollutant is a waste material that pollutes air, water or soil, and is the cause of pollution.Three factors determine the severity of a pollutant: its chemical nature, its concentration and its persistence. Some pollutants are biodegradable and therefore will not persist in the environment in the...
s because of contributions to ozone depletion
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
, global warming
Global warming
Global warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
, bioaccumulation
Bioaccumulation
Bioaccumulation refers to the accumulation of substances, such as pesticides, or other organic chemicals in an organism. Bioaccumulation occurs when an organism absorbs a toxic substance at a rate greater than that at which the substance is lost...
, and toxicity
Toxicity
Toxicity is the degree to which a substance can damage a living or non-living organisms. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell or an organ , such as the liver...
. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
The carbon–fluorine bond
Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens.- The carbon–fluorine bondCarbon–fluorine bondThe carbon–fluorine bond is a bond between carbon and fluorine that is a component of all organofluorine compounds. It is the strongest single bond in organic chemistry—and relatively short—due to its partial ionic character. The bond also strengthens and shortens as more fluorines are...
is one of the strongest in organic chemistry (an average bond energy around 480 kJ/mol). This is significantly stronger than the bonds of carbon with other halogens (an average bond energy of e.g. C-Cl bond is around 320 kJ/mol) and is one of the reasons why fluoroorganic compounds have high thermal and chemical stability. - The carbon–fluorine bondCarbon–fluorine bondThe carbon–fluorine bond is a bond between carbon and fluorine that is a component of all organofluorine compounds. It is the strongest single bond in organic chemistry—and relatively short—due to its partial ionic character. The bond also strengthens and shortens as more fluorines are...
is relatively short (around 1.4 Å). - The Van der Waals radiusVan der Waals radiusThe van der Waals radius, r, of an atom is the radius of an imaginary hard sphere which can be used to model the atom for many purposes. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms had a finite size and to...
of the fluorine substituent is only 1.47 Å, which is shorter than in any other substituent and is close to that of hydrogen (1.2 Å). This, together with the short bond length, is the reason why there is no steric strain in polyfluorinated compounds. This is another reason for their high thermal stability. In addition, the fluorine substituents in polyfluorinated compounds efficiently shield the carbon skeleton from possible attacking reagents. This is another reason for the high chemical stability of polyfluorinated compounds. - Fluorine has the highest electronegativityElectronegativityElectronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of all elements: 3.98. This causes the high dipole moment of C-F bond (1.41 D). - Fluorine has the lowest polarizability of all atoms: 0.56 10-24 cm3. This causes very weak dispersion forces between polyfluorinated molecules and is the reason for the often-observed boiling point reduction on fluorination as well as for the simultaneous hydrophobicity and lipophobicity of polyfluorinated compounds whereas other perhalogenated compounds are more lipophilicLipophilicLipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true...
.
In comparison to aryl chlorides and bromides, aryl fluorides form Grignard reagents only reluctantly. On the other hand, aryl fluorides, e.g. fluoroaniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s and fluorophenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s, often undergo nucleophilic substitution efficiently.
Hydrofluorocarbons
Hydrofluorocarbons, organic compounds that contain only one or a few fluorine atoms, are the more common type of organofluorine compounds. Used as refrigerants in place of the older chlorofluorocarbonChlorofluorocarbon
A chlorofluorocarbon is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons , which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon...
s such as Freon-12, they do not harm the ozone layer if they do not contain chlorine or bromine. However, their atmospheric concentrations are rapidly increasing, causing international concern about a different process: their rising contribution to anthropogenic radiative forcing emissions (i.e., greenhouse gas global warming
Global warming
Global warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
).
Fluorocarbons with few C-F bonds behave similarly to the parent hydrocarbons, but their reactivity can be altered significantly. For example, both uracil
Uracil
Uracil is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine, cytosine, and guanine. In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine.Uracil is a common and...
and 5-fluorouracil are colourless, high-melting crystalline solids, but the latter is a potent anti-cancer drug. The use of the C-F bond in pharmaceuticals is predicated on this altered reactivity. Several drugs and agrochemicals contain only one fluorine center or one trifluoromethyl
Trifluoromethyl
Trifluoromethyl is a functional group in organofluorines that has the formula -CF3. The naming of is group is derived from the methyl group , by replacing each hydrogen atom by a fluorine atom. The trifluomethyl group has a significant electronegativity that is often described as being...
group.
Fluorocarbons and fluorocarbon derivatives
FluorocarbonFluorocarbon
Fluorocarbons, sometimes referred to as perfluorocarbons or PFCs, are organofluorine compounds that contain only carbon and fluorine bonded together in strong carbon–fluorine bonds. Fluoroalkanes that contain only single bonds are more chemically and thermally stable than alkanes...
based molecules are more chemically and thermally stable than hydrocarbons, reflecting the relative inertness of the C-F bond. They are also relatively lipophobic. Because of the reduced intermolecular van der Waals interactions
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
, fluorocarbon-based compounds are often lubricants or are highly volatile. Gas soluble fluorocarbon liquids have medical applications. Fully fluorinated organic compounds, sometimes called perfluorocarbons or fluorocarbons, contain only carbon and fluorine. Chlorofluorocarbons (CFCs) are typically highly fluorinated.
Fluoropolymers
Polymeric organofluorine compounds are numerous and commercially significant. They range from fully fluorinated species, e.g. PTFE to partially fluorinated, e.g. polyvinylidene fluoridePolyvinylidene fluoride
Polyvinylidene fluoride, or PVDF is a highly non-reactive and pure thermoplastic fluoropolymer.PVDF is a specialty plastic material in the fluoropolymer family; it is used generally in applications requiring the highest purity, strength, and resistance to solvents, acids, bases and heat and low...
([CH2CF2]n) and polychlorotrifluoroethylene
Polychlorotrifluoroethylene
Polychlorotrifluoroethylene is a fluoropolymer with the molecular formula n. It is chemically related to PTFE....
([CFClCF2]n.
The structure of organofluorine compounds can be distinctive, again reflecting the polarizing character of the C-F bond. Fluorination affects packing of organofluorine molecules with hydrocarbons. As shown below, perfluorinated aliphatic compounds tend to segregate from hydrocarbons. This "like dissolves like effect" is related to the usefulness of fluorous phases and the use of PFOA in processing of fluoropolymers. In contrast to the aliphatic derivatives, perfluoroaromatic derivatives tend to form mixed phases with nonfluorinated aromatic compounds, resulting from donor-acceptor interactions between the pi-systems.
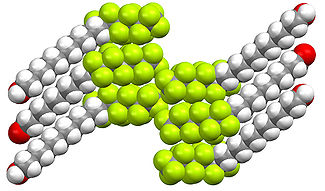
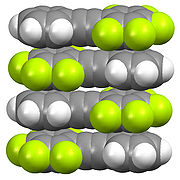
Fluorocarbenes
As indicated throughout this article, fluorine-substituents lead to reactivity that differs strongly from classical organic chemistry. The premier example is difluorocarbeneDifluorocarbene
Difluorocarbene is the chemical compound with formula CF2. It has a short half-life, 0.5 and 20 msec, in solution and in the gas phase, respectively...
, CF2, which is a singlet whereas carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
(CH2) has a triplet ground state. This difference is significant because difluorocarbene is a precursor to tetrafluoroethylene.
Methods for preparation of C-F bonds
Organofluorine compounds are prepared by numerous routes, depending on the degree and regiochemistry of fluorination sought and the nature of the precursors. The direct fluorination of hydrocarbons with F2, often diluted with N2, is useful for highly fluorinated compounds:- + → +
Such reactions however are often unselective and require care because hydrocarbons can uncontrollably "burn" in , analogous to the combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of hydrocarbon in . For this reason, alternative fluorination methodologies have been developed. Generally, such methods are classified into two classes.
Electrophilic fluorination
Electrophilic fluorination rely on sources of "F+". Often such reagents feature N-F bonds, for example F-TEDA-BF4F-TEDA-BF4
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis or Selectfluor® , a trademark of Air Products and Chemicals, is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the heterocycle DABCO®...
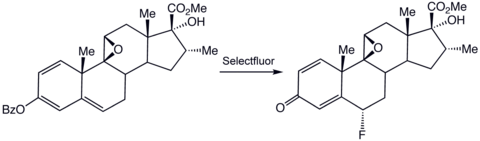
. Asymmetric fluorination, whereby only one of two possible enantiomeric products are generated from a prochiral substrate, rely on electrophilic fluorination reagents. Illustrative of this approach is the preparation of a precursor to anti-inflammatory agents:
Electrosynthetic methods
A specialized but important method of electrophilic fluorination involves electrosynthesisElectrosynthesis
Electrosynthesis in organic chemistry is the synthesis of chemical compounds in a electrochemical cell The main advantage of electrosynthesis over an ordinary redox reaction is avoidance of the potential wasteful other half-reaction and the ability to precisely tune the required potential...
. The method is mainly used to perfluorinate, i.e. replace all C–H bonds by C–F bonds. The hydrocarbon is dissolved or suspended in liquid HF, and the mixture is electrolyzed
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
at 5–6 V
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
using Ni anodes. The method was first demonstrated with the preparation of perfluoropyridine from pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
. Several variations of this technique have been described, including the use of molten potassium bifluoride
Potassium bifluoride
Potassium bifluoride is the inorganic compound with the formula KHF2. This colourless salt consists of the potassium cation and the bifluoride anion. The salt is used in etchant for glass...
or organic solvents.
Nucleophilic fluorination
The major alternative to electrophilic fluorination is, naturally, nucleophilic fluorination using reagents that are sources of "F-," for Nucleophilic displacement typically of chloride and bromide. Metathesis reactions employing alkali metalAlkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
fluorides are the simplest.
- + → + (M = Na, K, Cs)
The decomposition of aryldiazonium tetrafluoroborates in the Sandmeyer
Sandmeyer reaction
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts. It is named after the Swiss chemist Traugott Sandmeyer....
or Schiemann reaction
Schiemann reaction
The Schiemann reaction is a chemical reaction in which anilines are transformed to aryl fluorides via diazonium fluoroborates...
s exploit fluoroborates as F- sources.
- → + +
Although hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
may appear to be an unlikely nucleophile, it is the most common source of fluoride in the synthesis of organofluorine compounds. Such reactions are often catalysed by metal fluorides such as chromium trifluoride. 1,1,1,2-Tetrafluoroethane
1,1,1,2-Tetrafluoroethane
1,1,1,2-Tetrafluoroethane, R-134a, Genetron 134a, Suva 134a or HFC-134a, is a haloalkane refrigerant with thermodynamic properties similar to R-12 , but with less ozone depletion potential...
, a replacement for CFC’s, is prepared industrially using this approach:
- Cl2C=CClHTrichloroethyleneThe chemical compound trichloroethylene is a chlorinated hydrocarbon commonly used as an industrial solvent. It is a clear non-flammable liquid with a sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.The IUPAC name is...
+ 4 HF → F3CCFH2 + 3 HCl
Notice that this transformation entails two reaction types, metathesis (replacement of Cl- by F-) and hydrofluorination of an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
.
Deoxofluorination
Deoxofluorination agents effect the replacement hydroxylHydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
and carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups with one and two fluorides, respectively. One such reagent, useful for fluoride for oxide exchange in carbonyl compounds, is sulfur tetrafluoride
Sulfur tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula SF4. This species exists as a gas at standard conditions. It is a corrosive species that releases dangerous HF upon exposure to water or moisture...
:
- RCO2H + SF4 → RCF3 + SO2Sulfur dioxideSulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
+ HF
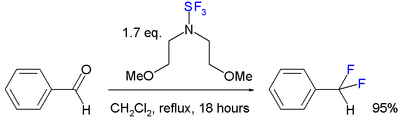
Alternates to SF4 include the diethylaminosulfur trifluoride
Diethylaminosulfur trifluoride
Diethylaminosulfur trifluoride is the organosulfur compound with the formula Et2NSF3. This liquid is a fluorinating reagent used for the synthesis of organofluorine compounds...
(DAST, NEt2SF3) and bis(2-methoxyethyl)aminosulfur trifluoride (deoxo-fluor). These organic reagents are easier to handle and more selective:
From fluorinated building blocks
Many organofluorine compounds are generated from reagents that deliver perfluoroalkyl and perfluoroaryl groups. (Trifluoromethyl)trimethylsilane, CF3Si(CH3)3, is used as a source of the trifluoromethylTrifluoromethyl
Trifluoromethyl is a functional group in organofluorines that has the formula -CF3. The naming of is group is derived from the methyl group , by replacing each hydrogen atom by a fluorine atom. The trifluomethyl group has a significant electronegativity that is often described as being...
group, for example. Among the available fluorinated building blocks are CF3X (X = Br, I), C6F5Br, and C3F7I. These species form Grignard reagents that then can be treated with a variety of electrophile
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s. The development of fluorous technologies (see below, under solvents) is leading to the development of reagents for the introduction of "fluorous tails."
A special but significant application of the fluorinated building block approach is the synthesis of tetrafluoroethylene
Tetrafluoroethylene
Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:...
, which is produced on a large-scale industrially via the intermediacy of difluorocarbene. The process begins with the thermal (600-800 °C) dehydrochlorination of chlorodifluoromethane
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon . This colorless gas is better known as HCFC-22, or R-22. It was once commonly used as a propellant and in air conditioning applications...
:
- CHClF2 → CF2 + HCl
- 2 CF2 → C2F4
Sodium fluorodichloroacetate (CAS# 2837-90-3) is used to generate chlorofluorocarbene, for cyclopropanations.
18F-Delivery methods
The usefulness of fluorine-containing radiopharmaceuticals in 18F-positron emission tomographyPositron emission tomography
Positron emission tomography is nuclear medicine imaging technique that produces a three-dimensional image or picture of functional processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide , which is introduced into the body on a...
has motivated the development of new methods for forming C-F bonds. Because of the short half-life of 18F, these syntheses must be highly efficient, rapid, and easy. Illustrative of the methods is the preparation of fluoride-modified glucose by displacement of a triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
by a labeled fluoride nucleophile:
Applications
Organofluorine chemistry impacts many areas of everyday life and technology. The C-F bond is found in pharmaceuticals, agrichemicalAgrichemical
Agrochemical , a contraction of agricultural chemical, is a generic term for the various chemical products used in agriculture. In most cases, agrichemical refers to the broad range of pesticides, including insecticides, herbicides, and fungicides...
s, fluoropolymers, refrigerants, surfactants, anesthetics, oil-repellents
Lipophobicity
Lipophobicity, also sometimes called lipophobia, is a chemical property of chemical compounds which means "fat rejection", literally "fear of fat". Lipophobic compounds are those not soluble in lipids or other non-polar solvents...
, catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
, and water-repellents
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
, among others.
Pharmaceuticals and agrochemicals
The carbon-fluorine bond is commonly found in pharmaceuticals and agrochemicals because it is generally metabolically stable and fluorine acts as a bioisostereBioisostere
In medicinal chemistry, bioisosteres are substituents or groups with similar physical or chemical properties which produce broadly similar biological properties to a chemical compound. In drug design, the purpose of exchanging one bioisostere for another is to enhance the desired biological or...
of the hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom. An estimated one fifth of pharmaceuticals contain fluorine, including several of the top drugs. Examples include 5-fluorouracil, flunitrazepam
Flunitrazepam
Flunitrazepam is marketed as a potent hypnotic, sedative, anticonvulsant, anxiolytic, amnestic, and skeletal muscle relaxant drug most commonly known as Rohypnol...
(Rohypnol), fluoxetine
Fluoxetine
Fluoxetine is an antidepressant of the selective serotonin reuptake inhibitor class. It is manufactured and marketed by Eli Lilly and Company...
(Prozac), paroxetine
Paroxetine
Paroxetine is an SSRI antidepressant. Marketing of the drug began in 1992 by the pharmaceutical company SmithKline Beecham, now GlaxoSmithKline...
(Paxil), ciprofloxacin
Ciprofloxacin
Ciprofloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class.It is a second-generation fluoroquinolone antibacterial. It kills bacteria by interfering with the enzymes that cause DNA to rewind after being copied, which stops synthesis of DNA and of...
(Cipro), mefloquine
Mefloquine
Mefloquine hydrochloride is an orally administered medication used in the prevention and treatment of malaria. Mefloquine was developed in the 1970s at the United States Department of Defense's Walter Reed Army Institute of Research as a synthetic analogue of quinine...
, and fluconazole
Fluconazole
Fluconazole is a triazole antifungal drug used in the treatment and prevention of superficial and systemic fungal infections. In a bulk powder form, it appears as a white crystalline powder, and it is very slightly soluble in water and soluble in alcohol. It is commonly marketed under the trade...
. Fluorine-substituted ethers are volatile anesthetics, including the commercial products methoxyflurane
Methoxyflurane
Methoxyflurane is a halogenated ether that was in clinical use as an volatile inhalational anesthetic from its introduction by Joseph F. Artusio et al in 1960 until around 1974. It was first synthesized in the late 1940s by William T...
, enflurane
Enflurane
Enflurane is a halogenated ether that was commonly used for inhalational anesthesia during the 1970s and 1980s. Developed by Ross Terrell in 1963, it was first used clinically in 1966....
, isoflurane
Isoflurane
Isoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
, sevoflurane
Sevoflurane
Sevoflurane , also called fluoromethyl hexafluoroisopropyl ether, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used for induction and maintenance of general anesthesia. Together with desflurane, it is replacing isoflurane and halothane in modern anesthesiology...
and desflurane
Desflurane
Desflurane is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane and isoflurane, it is a racemic mixture of and optical isomers...
. Fluorocarbon anesthetics reduce the hazard of flammability with diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
and cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
. Perfluorinated alkanes are used as blood substitute
Blood substitute
A blood substitute is a substance used to mimic and fulfill some functions of biological blood, usually in the oxygen-carrying sense...
s.
Fluorosurfactants
Fluorosurfactants, which have a polyfluorinated "tail" and a hydrophilic "head", serve surfactants because they concentrate at the liquid-air interface due to their lipophobicityLipophobicity
Lipophobicity, also sometimes called lipophobia, is a chemical property of chemical compounds which means "fat rejection", literally "fear of fat". Lipophobic compounds are those not soluble in lipids or other non-polar solvents...
. Fluorosurfactants have low surface energies and dramatically lower surface tension. The fluorosurfactants perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid
Perfluorooctanoic acid
Perfluorooctanoic acid , also known as C8 and perfluorooctanoate, is a synthetic, stable perfluorinated carboxylic acid and fluorosurfactant. One industrial application is as a surfactant in the emulsion polymerization of fluoropolymers. It has been used in the manufacture of such prominent...
(PFOA) are two of the most studied because of their ubiquity, toxicity, and long residence times in humans and wildlife.
Solvents
Fluorinated compounds often display distinct solubility properties. DichlorodifluoromethaneDichlorodifluoromethane
Dichlorodifluoromethane , is a colorless gas, and usually sold under the brand name Freon-12, is a chlorofluorocarbon halomethane , used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in the United States along with many other...
and chlorodifluoromethane
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon . This colorless gas is better known as HCFC-22, or R-22. It was once commonly used as a propellant and in air conditioning applications...
were widely used refrigerants. CFCs have potent ozone depletion
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
potential due to the homolytic cleavage
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
of the carbon-chlorine bonds; their use is largely prohibited by the Montreal Protocol
Montreal Protocol
The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances believed to be responsible for ozone depletion...
. Hydrofluorocarbons (HFCs), such as tetrafluoroethane, serve as CFC replacements because they do not catalyze ozone depletion. Oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
exhibits a high solubility in perfluorocarbon compounds, reflecting again on their lipophilicity. Perfluorodecalin
Perfluorodecalin
Perfluorodecalin is a fluorocarbon, a derivative of decalin in which all of the hydrogen atoms are replaced by fluorine atoms. It is chemically and biologically inert, and stable up to 400°C. Several applications make use of its ability to dissolve gases....
has been demonstrated as a blood substitutes
Blood substitutes
A blood substitute is a substance used to mimic and fulfill some functions of biological blood, usually in the oxygen-carrying sense...
, transporting oxygen to the lungs.
The solvent 1,1,1,2-tetrafluoroethane
1,1,1,2-Tetrafluoroethane
1,1,1,2-Tetrafluoroethane, R-134a, Genetron 134a, Suva 134a or HFC-134a, is a haloalkane refrigerant with thermodynamic properties similar to R-12 , but with less ozone depletion potential...
has been used for extraction
Liquid-liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid...
of natural products such as taxol, evening primrose oil, and vanillin
Vanillin
Vanillin is a phenolic aldehyde, an organic compound with the molecular formula C8H8O3. Its functional groups include aldehyde, ether, and phenol. It is the primary component of the extract of the vanilla bean. It is also found in Leptotes bicolor, roasted coffee and the Chinese red pine...
. 2,2,2-trifluoroethanol
2,2,2-Trifluoroethanol
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic...
is an oxidation-resistant polar solvent.
Organofluorine reagents
The development of organofluorine chemistry has contributed many reagents of value beyond organofluorine chemistry. Triflic acid (CF3SO3H) and trifluoroacetic acidTrifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
(CF3CO2H) are useful throughout organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. Their strong acidity is attributed to the electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of the trifluoromethyl
Trifluoromethyl
Trifluoromethyl is a functional group in organofluorines that has the formula -CF3. The naming of is group is derived from the methyl group , by replacing each hydrogen atom by a fluorine atom. The trifluomethyl group has a significant electronegativity that is often described as being...
group that stabilizes the negative charge. The triflate-group (the conjugate base of the triflic acid) is a good leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
in substitution reactions.
Fluorous phases
Of topical interest in the area of "Green Chemistry," highly fluorinated substituents, e.g. perfluorohexyl (C6F13) confer distinctive solubility properties to molecules, which facilitates purification of products in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. This area, described as "fluorous
Fluorous
Fluorous chemistry is chemistry that uses perfluorinated compounds, commonly the fluorosurfactant perfluorooctanoic acid . Due to the extreme electronegativity of fluorine, perfluorinated compounds have unique physical properties which are useful in organic synthesis and separation methods such as...
chemistry," exploits the concept of like-dissolves-like in the sense that fluorine-rich compounds dissolve preferentially in fluorine-rich solvents. Because of the relative inertness of the C-F bond, such fluorous phases are compatible with even harsh reagents. This theme has spawned techniques of “fluorous tagging’’ and ‘‘fluorous protection’’. Illustrative of fluorous technology is the use of fluoroalkyl-substituted tin hydrides for reductions, the products being easily separated from the spent tin reagent by extraction using fluorinated solvents.
Hydrophobic fluorinated ionic liquid
Ionic liquid
An ionic liquid is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ILs are largely made...
s, such as organic salts of bistriflimide
Bistriflimide
Bistriflimide, systematically known as bissulfonimide and colloquially known as TFSI is a non-coordinating anion with the chemical formula of [2N]-. The anion is widely used in ionic liquids, since it is less toxic and more stable than more "traditional" counterions such as tetrafluoroborate...
or hexafluorophosphate
Hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . This octahedral species is isoelectronic with sulfur hexafluoride, SF6, and is valence isoelectronic with the highly stable superacid anion fluoroantimonate . As a non-coordinating anion, it is a poor nucleophile...
, can form phases that are insoluble in both water and organic solvents, producing multiphasic liquid
Multiphasic liquid
A multiphasic liquid is a mixture consisting of more than two immiscible liquid phases. Biphasic mixtures consisting of two immiscible phases are very common and usually consist of an organic solvent and an aqueous phase...
s.
Organofluorine ligands in transition metal chemistry
Organofluorine ligands have long been featured in organometallicOrganometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
and coordination chemistry. One advantage to F-containing ligands is the convenience of 19F NMR spectroscopy
Fluorine-19 NMR
Fluorine-19 nuclear magnetic resonance is an analytical technique. 19F has a spin of 1/2, and a relative abundance of 100 % and a high magnetogyric ratio, making measurements very fast . Integrals are reliable due to the lack of a nuclear Overhauser effect...
for monitoring reactions. The organofluorine compounds can serve as a "sigma-donor ligand," as illustrated by the titanium(III) derivative [(C5Me5)2Ti(FC6H5)]BPh4. Most often, however, fluorocarbon substituents are used to enhance the Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
ity of metal centers. A premier example is “Eufod
Eufod
EuFOD is the chemical compound with the formula Eu3, also called Eu3. This coordination compound is used primarily as a shift reagent in NMR spectroscopy...
,” a coordination complex of europium(III) that features a perfluoroheptyl modified acetylacetonate ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. This and related species are useful in organic synthesis and as "shift reagents" in NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
.
.png)
Materials science
Materials science is an interdisciplinary field applying the properties of matter to various areas of science and engineering. This scientific field investigates the relationship between the structure of materials at atomic or molecular scales and their macroscopic properties. It incorporates...
overlap, the fluorination of organic ligands is used to tune the properties of component molecules. For example, the degree and regiochemistry of fluorination of metalated 2-phenylpyridine ligands in platinum(II) complexes significantly modifies the emission properties of the complexes.
The coordination chemistry of organofluorine ligands also embraces fluorous technologies. For example, triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
has been modified by attachment of perfluoroalkyl substituents that confer solubility in perfluorohexane
Perfluorohexane
Perfluorohexane or tetradecafluorohexane, is a fluorocarbon. It is a derivative of hexane in which all of the hydrogen atoms are replaced by fluorine atoms. It is used in one formulation of the electronic cooling liquid/insulator Fluorinert for low temperature applications due to its low boiling...
as well as supercritical carbon dioxide
Supercritical carbon dioxide
Supercritical carbon dioxide is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.Carbon dioxide usually behaves as a gas in air at STP or as a solid called dry ice when frozen...
. As a specific example, [(C8F17C3H6-4-C6H4)3P.
C-F bond activation
An active area of organometallic chemistry encompasses the scission of C-F bonds by transition metal-based reagents. Both stoichiometric and catalytic reactions have been developed and are of interest from the perspectives of organic synthesis and remediation of xenochemicals. C-F bond activation has been classified as follows “(i) oxidative addition of fluorocarbon, (ii) M–C bond formation with HF elimination, (iii) M–C bond formation with fluorosilane elimination, (iv) hydrodefluorination of fluorocarbon with M–F bond formation, (v) nucleophilic attack on fluorocarbon, and (vi) defluorination of fluorocarbon.” An illustrative metal-mediated C-F activation reaction is the defluorination of fluorohexane by a zirconium dihydrideHydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
, an analogue of Schwartz's reagent
Schwartz's Reagent
Schwartz's reagent is the common name for the chemical compound with the formula 2ZrHCl, sometimes described zirconocene hydrochloride or zirconocene chloride hydride and is named after Jeffrey Schwartz, who is currently a professor in Chemistry at Princeton University...
:2ZrH2 + 1-FC6H13 → (C5Me5)2ZrH(F) + C6H14
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
Fluorocarbon anions in Ziegler-Natta catalysis
Fluorine-containing compounds are often featured in noncoordinating or weakly coordinating anions. Both tetrakis(pentafluorophenyl)borate, B(C6F5)4-, and the related tetrakis(3,5-bis(trifluoromethyl)phenyl)borate, are useful in Ziegler-Natta catalysis and related alkene polymerization methodologies. The fluorinated substituents render the anions weakly basic and enhance the solubility in weakly basic solvents, which are compatible with strong Lewis acids.Materials science
Organofluorine compounds enjoy many niche applications in materials scienceMaterials science
Materials science is an interdisciplinary field applying the properties of matter to various areas of science and engineering. This scientific field investigates the relationship between the structure of materials at atomic or molecular scales and their macroscopic properties. It incorporates...
. With a low coefficient of friction, fluid fluoropolymers are used as specialty lubricants. Fluorocarbon-based greases are used in demanding applications. Representative products include Fomblin and Krytox
Krytox
Krytox is a trademarked name of a family of high-performance synthetic lubricants with a variety of applications. Invented by researchers at DuPont, it is a colourless polymer containing ether functionality...
, manufactured by Solvay Solexis and DuPont
DuPont
E. I. du Pont de Nemours and Company , commonly referred to as DuPont, is an American chemical company that was founded in July 1802 as a gunpowder mill by Eleuthère Irénée du Pont. DuPont was the world's third largest chemical company based on market capitalization and ninth based on revenue in 2009...
, respectively. Certain firearm lubricants such as "Tetra Gun" contain fluorocarbons. Capitalizing on their nonflammability, fluorocarbons are used in fire fighting foam. Organofluorine compounds are components of liquid crystal display
Liquid crystal display
A liquid crystal display is a flat panel display, electronic visual display, or video display that uses the light modulating properties of liquid crystals . LCs do not emit light directly....
s. The polymeric analogue of triflic acid, nafion
Nafion
Nafion is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Walther Grot of DuPont. It is the first of a class of synthetic polymers with ionic properties which are called ionomers...
is a solid acid that is used as the membrane in most low temperature fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s. The bifunctional monomer 4,4'-difluorobenzophenone
4,4'-Difluorobenzophenone
4,4’-Difluorobenzophenone is an organic compound with the formula of 2CO. This a colorless solid is commonly used as a precursor to PEEK, or polyetherether ketone, a so-called high performance polymer...
is a precursor to PEEK
PEEK
Polyether ether ketone is a colourless organic polymer thermoplastic used in engineering applications.-Synthesis:PEEK polymers are obtained by step-growth polymerization by the dialkylation of bisphenolate salts. Typical is the reaction of 4,4'-difluorobenzophenone with the disodium salt of...
-class polymers.
Biosynthesis of organofluorine compounds
In contrast to the many naturally-occurring organic compounds containing the heavier halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s, chloride, bromide, and iodide, only a handful of biologically synthesized carbon-fluorine bonds are known. The most common natural organofluorine species is fluoroacetate
Fluoroacetic acid
Fluoroacetic acid is a compound with formula H. It is between acetic acid and trifluoroacetic acid....
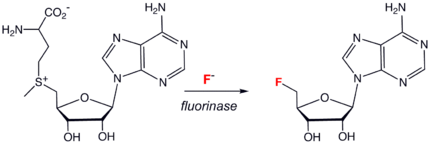
, a toxin found in a few species of plants. Others include fluorooleic acid, fluoroacetone, nucleocidin (4’-fuoro-5’-O-sulphamoyladenosine), fluorothreonine, and 2-fluorocitrate. Several of these species are probably biosynthesized from fluoroacetaldehyde. The enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
fluorinase catalyzed the synthesis of 5'-fluoro-5-deoxyadenosine (see scheme to right).
History
Organofluorine chemistry began in the 1800s with the development of organic chemistry as a whole. The first organofluorine compounds were prepared by metathesis reactions using antimony trifluorideAntimony trifluoride
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swart's reagent, is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid...
as the F- source. The nonflammability and nontoxicity of the chlorofluorocarbon
Chlorofluorocarbon
A chlorofluorocarbon is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane. A common subclass are the hydrochlorofluorocarbons , which contain hydrogen, as well. They are also commonly known by the DuPont trade name Freon...
s CCl3F and CCl2F2 attracted industrial attention in the 1920s. In the 1930s, scientists at duPont discovered polytetrafluoroethylene. Subsequent major developments, especially in the US, benefited from expertise gained in the production of uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
. Starting in the late 1940’s, a series of electrophilic fluorinating methodologies were introduced, beginning with CoF3. About this time, electrochemical fluorination ("electrofluorination") was announced, having been developed in the 1930s with the goal of generating highly stable perfluorinated materials compatible with uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
. These new methodologies allowed the synthesis of C-F bonds without using elemental fluorine and without relying on metathetical methods. In 1957, the anticancer activity of 5-fluorouracil was described. This report provided one of the first examples of rational design of drugs. This discovery sparked a surge of interest in fluorinated pharmaceuticals and agrichemicals. The discovery of the noble gas compound
Noble gas compound
Noble gas compounds are chemical compounds that include an element from Group 18 of the periodic table, the noble gases.-History and background:...
s, e.g. XeF4, provided a host of new reagents starting in the early 1960’s. In the 1970s, fluorodeoxyglucose
Fluorodeoxyglucose
Fludeoxyglucose or fluorodeoxyglucose , commonly abbreviated 18F-FDG or FDG, is a radiopharmaceutical used in the medical imaging modality positron emission tomography...
was established as a useful reagent in 18F positron emission tomography
Positron emission tomography
Positron emission tomography is nuclear medicine imaging technique that produces a three-dimensional image or picture of functional processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide , which is introduced into the body on a...
. In Nobel Prize-winning work, CFC’s were shown to contribute to the depletion of atmospheric ozone. This discovery alerted the world to the negative consequences of organofluorine compounds and motivated the development of new routes to organofluorine compounds. In 2002, the first C-F bond-forming enzyme, fluorinase, was reported.