
Carbon–fluorine bond
Encyclopedia

Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
between carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
that is a component of all organofluorine compounds. It is the strongest single bond in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
—and relatively short—due to its partial ionic
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
. As such, fluoroalkanes
Fluorocarbon
Fluorocarbons, sometimes referred to as perfluorocarbons or PFCs, are organofluorine compounds that contain only carbon and fluorine bonded together in strong carbon–fluorine bonds. Fluoroalkanes that contain only single bonds are more chemically and thermally stable than alkanes...
like tetrafluoromethane
Tetrafluoromethane
Tetrafluoromethane, also known as carbon tetrafluoride, is the simplest fluorocarbon . It has a very high bond strength due to the nature of the carbon–fluorine bond. It can also be classified as a haloalkane or halomethane...
(carbon tetrafluoride) are some of the most unreactive
Chemical stability
Chemical stability when used in the technical sense in chemistry, means thermodynamic stability of a chemical system.Thermodynamic stability occurs when a system is in its lowest energy state, or chemical equilibrium with its environment. This may be a dynamic equilibrium, where individual atoms...
organic compounds.
Electronegativity and bond strength
The high electronegativityElectronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of fluorine (4.0 for F vs. 2.5 for carbon) gives the carbon–fluorine bond a significant polarity
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
/dipole moment
Electron electric dipole moment
The electron electric dipole moment de is an intrinsic property of an electron such that the potential energy is linearly related to the strength of the electric field: U=de·E. Within the standard model of elementary particle physics, such a dipole is predicted to be non-zero but very small, at...
. The electron density is concentrated around the fluorine, leaving the carbon relatively electron poor. This introduces ionic character to the bond through partial charges (Cδ+—Fδ−). The partial charges on the fluorine and carbon are attractive, contributing to the unusual bond strength of the carbon–fluorine bond. The bond is labeled as "the strongest in organic chemistry," because fluorine forms the strongest single bond to carbon. Carbon–fluorine bonds can have a bond dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
(BDE) of up to 544 kJ/mol. The BDE (strength of the bond) is higher than other carbon–halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
and carbon–hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
bonds. For example, the molecule represented by CH3X has a BDE of 115 kcal/mol for carbon–fluorine while values of 104.9, 83.7, 72.1, and 57.6 kcal/mol represent carbon–X bonds to hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
, and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, respectively.
Bond length
The carbon–fluorine bond length is typically about 1.35 ångströmÅngström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
(1.39 Å in fluoromethane
Fluoromethane
Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane plus fluorine, minus a hydrogen...
). It is shorter than any other carbon–halogen bond, and shorter than single carbon–nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and carbon–oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
bonds, despite fluorine having a larger atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
. The short length of the bond can also be attributed to the ionic character/electrostatic attractions between the partial charges on carbon and fluorine. The carbon–fluorine bond length varies by several hundredths of an angstrom depending on the hybridization of the carbon atom and the presence of other substituents on the carbon or even in atoms farther away. These fluctuations can be used as indication of subtle hybridization changes and stereoelectronic interactions. The table below shows how the average bond length varies in different bonding environments (carbon atoms are sp3-hybridized unless otherwise indicated for sp2 or aromatic carbon).
| Bond | Mean bond length (Å) |
|---|---|
| CCH2F, C2CHF | 1.399 |
| C3CF | 1.428 |
| C2CF2, H2CF2, CCHF2 | 1.349 |
| CCF3 | 1.346 |
| FCNO2 | 1.320 |
| FCCF | 1.371 |
| Csp2F | 1.340 |
| CarF | 1.363 |
| FCarCarF | 1.340 |
The variability in bond lengths and the shortening of bonds to fluorine due to their partial ionic character are also observed for bonds between fluorine and other elements, and have been a source of difficulties with the selection of an appropriate value for the covalent radius of fluorine
Covalent radius of fluorine
The covalent radius of fluorine is a measure of the size of a fluorine atom; it is approximated at about 60 picometres.Since fluorine is a relatively small atom with a large electronegativity, its covalent radius is difficult to evaluate. The covalent radius is defined as half the bond lengths...
. Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
originally suggested 64 pm, but that value was eventually replaced by 72 pm, which is half of the fluorine–fluorine bond length. However, 72 pm is too long to be representative of the lengths of the bonds between fluorine and other elements, so values between 54 pm and 60 pm have been suggested by other authors.
Bond strength effect of geminal bonds
With increasing number of fluorine atoms on the same (geminalGeminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
) carbon the other bonds become stronger and shorter. This can be seen by the changes in bond length and strength (BDE) for the fluoromethane series, as shown on the table below; also, the partial charges (qC and qF) on the atoms change within the series. The partial charge on carbon becomes more positive as fluorines are added, increasing the electrostatic interactions, and ionic character, between the fluorines and carbon.
| Compound | C-F bond length (Å) | BDE (kcal/mol) | qC | qF |
|---|---|---|---|---|
| CH3F | 1.385 | 109.9 ± 1 | 0.01 | −0.23 |
| CH2F2 | 1.357 | 119.5 | 0.40 | −0.23 |
| CHF3 | 1.332 | 127.5 | 0.56 | −0.21 |
| CF4 | 1.319 | 130.5 ± 3 | 0.72 | −0.18 |
Gauche effect
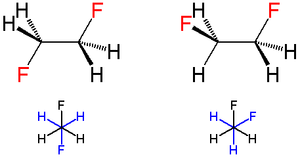
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
(i.e., adjacent) carbons, as in 1,2-difluoroethane (H2FCCFH2), the gauche conformer is more stable than the anti conformer—this is the opposite of what would normally be expected and to what is observed for most 1,2-disubstituted ethanes; this phenomenon is known as the gauche effect. In 1,2-difluoroethane, the gauche conformation is more stable than the anti conformation by 2.4 to 3.4 kJ/mole in the gas phase. This effect is not unique to the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
fluorine, however; the gauche effect is also observed for 1,2-dimethoxyethane. A related effect is the cis effect in alkenes. For instance, the cis isomer of 1,2-difluoroethylene is more stable than the trans isomer.
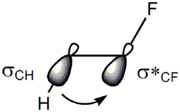
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
and bent bonds. In the hyperconjugation model, the donation of electron density from the carbon–hydrogen σ bonding orbital to the carbon–fluorine σ* antibonding orbital is considered the source of stabilization in the gauche isomer. Due to the greater electronegativity of fluorine, the carbon–hydrogen σ orbital is a better electron donor than the carbon–fluorine σ orbital, while the carbon–fluorine σ* orbital is a better electron acceptor than the carbon–hydrogen σ* orbital. Only the gauche conformation allows good overlap between the better donor and the better acceptor.
Key in the bent bond explanation of the gauche effect in difluoroethane is the increased p orbital character of both carbon–fluorine bonds due to the large electronegativity of fluorine. As a result, electron density builds up above and below to the left and right of the central carbon–carbon bond. The resulting reduced orbital overlap can be partially compensated when a gauche conformation is assumed, forming a bent bond. Of these two models, hyperconjugation is generally considered the principal cause behind the gauche effect in difluoroethane.
Spectroscopy
The carbon–fluorine bond stretching appears in the infrared spectrum between 1000 and 1360 cm−1. The wide range is due to the sensitivity of the stretching frequency to other substituents in the molecule. Monofluorinated compounds have a strong band between 1000 and 1110 cm−1; with more than one fluorine atoms, the band splits into two bands, one for the symmetric mode and one for the asymmetric. The carbon–fluorine bands are so strong that they may obscure any carbon–hydrogen bands that might be present.Organofluorine compounds can also be characterized using NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, using carbon-13
Carbon-13
Carbon-13 is a natural, stable isotope of carbon and one of the environmental isotopes. It makes up about 1.1% of all natural carbon on Earth.- Detection by mass spectrometry :...
, fluorine-19 (the only natural fluorine isotope), or hydrogen-1 (if present). The chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
s in 19F NMR
Fluorine-19 NMR
Fluorine-19 nuclear magnetic resonance is an analytical technique. 19F has a spin of 1/2, and a relative abundance of 100 % and a high magnetogyric ratio, making measurements very fast . Integrals are reliable due to the lack of a nuclear Overhauser effect...
appear over a very wide range, depending on the degree of substitution and functional group. The table below shows the ranges for some of the major classes.
| Type of Compound | Chemical Shift Range (ppm) Relative to neat CFCl3 |
|---|---|
| F–C=O | −70 to −20 |
| CF3 | +40 to +80 |
| CF2 | +80 to +140 |
| CF | +140 to +250 |
| ArF | +80 to +170 |

