
Supercritical carbon dioxide
Encyclopedia
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
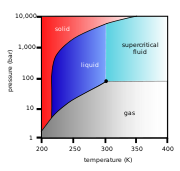
where it is held at or above its critical temperature and critical pressure.
Carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
usually behaves as a gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
in air at STP
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
or as a solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
called dry ice
Dry ice
Dry ice, sometimes referred to as "Cardice" or as "card ice" , is the solid form of carbon dioxide. It is used primarily as a cooling agent. Its advantages include lower temperature than that of water ice and not leaving any residue...
when frozen. If the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
are both increased from STP
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
to be at or above the critical point
Critical point (thermodynamics)
In physical chemistry, thermodynamics, chemistry and condensed matter physics, a critical point, also called a critical state, specifies the conditions at which a phase boundary ceases to exist...
for carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, it can adopt properties midway between a gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
and a liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
. More specifically, it behaves as a supercritical fluid
Supercritical fluid
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
above its critical temperature (31.1 °C) and critical pressure (72.9 atm (7.4 MPa)), expanding to fill its container like a gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
but with a density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
like that of a liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
.
Supercritical CO2 is becoming an important commercial and industrial solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
due to its role in chemical extraction
Extraction (chemistry)
Extraction in chemistry is a separation process consisting in the separation of a substance from a matrix. It may refer to Liquid-liquid extraction, and Solid phase extraction....
in addition to its low toxicity and environmental impact. The relatively low temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of the process and the stability of CO2 also allows most compounds to be extracted with little damage or denaturing
Denaturation (food)
Food is deliberately denatured when a substance, known as a denaturant, is added to render the food unpleasant to consume or poisonous. Aversive agents—primarily bitterants and pungent agents—are used to produce an unpleasant flavor. For example, the bitterant denatonium might be added to food used...
. In addition, the solubility of many extracted compounds in CO2 vary with pressure, permitting selective extractions.
Solvent
Carbon dioxide is gaining popularity amongst coffeeCoffee
Coffee is a brewed beverage with a dark,init brooo acidic flavor prepared from the roasted seeds of the coffee plant, colloquially called coffee beans. The beans are found in coffee cherries, which grow on trees cultivated in over 70 countries, primarily in equatorial Latin America, Southeast Asia,...
manufacturers looking to move away from some of the classic decaffeinating solvents of the past, many of which lead to public outcry because of real or perceived dangers related to their use in food preparation. Supercritical CO2 is forced through the green coffee beans and then they are sprayed with water at high pressure to remove the caffeine. The caffeine can then be isolated for resale (e.g. to the pharmaceutical industry or to beverage manufacturers) by passing the water through activated charcoal filters
Activated carbon
Activated carbon, also called activated charcoal, activated coal or carbo activatus, is a form of carbon that has been processed to make it extremely porous and thus to have a very large surface area available for adsorption or chemical reactions.The word activated in the name is sometimes replaced...
or by distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
, crystallization
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
or reverse osmosis
Reverse osmosis
Reverse osmosis is a membrane technical filtration method that removes many types of large molecules and ions from solutions by applying pressure to the solution when it is on one side of a selective membrane. The result is that the solute is retained on the pressurized side of the membrane and...
.
Supercritical carbon dioxide can also be used as a more environmentally friendly solvent for dry cleaning
Dry cleaning
Dry cleaning is any cleaning process for clothing and textiles using a chemical solvent other than water. The solvent used is typically tetrachloroethylene , abbreviated "perc" in the industry and "dry-cleaning fluid" by the public...
as compared to more traditional solvents such as hydrocarbons and perchloroethylene.
Supercritical carbon dioxide is used as the extraction solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
for creation of essential oils and other herbal distillates. Its main advantages over solvents such as hexane
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
in this process are that it is non toxic and non-flammable. Furthermore, separation of the reaction components from the starting material is much simpler than with traditional organic solvents, merely by allowing it to evaporate into the air recycling it by condensation into a cold recovery vessel. Its advantage over steam distillation
Steam distillation
Steam distillation is a special type of distillation for temperature sensitive materials like natural aromatic compounds....
is that it is used at a lower temperature, which can separate the plant waxes from the oils.
In laboratories
Laboratory
A laboratory is a facility that provides controlled conditions in which scientific research, experiments, and measurement may be performed. The title of laboratory is also used for certain other facilities where the processes or equipment used are similar to those in scientific laboratories...
, supercritical carbon dioxide is used as an extraction solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, e.g., in determination of Total Recoverable Hydrocarbons from soils, sediments, fly-ash, and other media, and determination of PAHs in soil and solid wastes. Supercritical fluid extraction has also been used in determination of hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
components in water.
Processes which use supercritical carbon dioxide to produce micro and nano
Nano
Nano- is a prefix meaning a billionth. Used primarily in the metric system, this prefix denotes a factor of 10−9 or . It is frequently encountered in science and electronics for prefixing units of time and length, such as 30 nanoseconds , 100 nanometres or in the case of electrical capacitance,...
scale particles, often for pharmaceutical uses, are currently being developed. The gas antisolvent process, rapid expansion of supercritical solutions, and supercritical antisolvent precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
(as well as several related methods) have been shown to process a variety of substances into particles.
Manufactured products
Environmentally beneficial, low-cost substitutes for rigid thermoplasticThermoplastic
Thermoplastic, also known as a thermosoftening plastic, is a polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently...
and fired ceramic
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
, are made using supercritical carbon dioxide as a chemical reagent. The supercritical carbon dioxide in these processes is reacted with the alkaline components of fully hardened hydraulic cement or gypsum
Gypsum
Gypsum is a very soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is found in alabaster, a decorative stone used in Ancient Egypt. It is the second softest mineral on the Mohs Hardness Scale...
plaster
Plaster
Plaster is a building material used for coating walls and ceilings. Plaster starts as a dry powder similar to mortar or cement and like those materials it is mixed with water to form a paste which liberates heat and then hardens. Unlike mortar and cement, plaster remains quite soft after setting,...
to form various carbonates. The primary byproduct is water.
Supercritical carbon dioxide is also used in the foaming of polymers. Many corporations utilize supercritical carbon dioxide to saturate the polymer with solvent (carbon dioxide). Upon depressurization and heating the carbon dioxide rapidly expands, causing voids within the polymer matrix, i.e., creating a foam. Research is also ongoing at many universities in the production of microcellular foams using supercritical carbon dioxide.
Working fluid
There is considerable work being done to develop a supercritical carbon dioxide closed-Brayton-cycleBrayton cycle
The Brayton cycle is a thermodynamic cycle that describes the workings of the gas turbine engine, basis of the airbreathing jet engine and others. It is named after George Brayton , the American engineer who developed it, although it was originally proposed and patented by Englishman John Barber...
gas turbine
Gas turbine
A gas turbine, also called a combustion turbine, is a type of internal combustion engine. It has an upstream rotating compressor coupled to a downstream turbine, and a combustion chamber in-between....
to operate at temperatures near 550 °C. This is a significant usage, which could have large implications for bulk thermal and nuclear generation of electricity, because the supercritical properties of carbon dioxide at above 500 °C and 20 MPa enable very high thermal efficiencies, approaching 45 percent. This could increase the electrical power produced per unit of fuel required by 40 percent or more. Given the volume of polluting fuels used in producing electricity, the environmental impact of cycle efficiency increases would be significant.
Supercritical carbon dioxide is also an important emerging natural refrigerant, being used in new, low carbon solutions for domestic heat pumps. These systems are undergoing continuous development with supercritical carbon dioxide heat pumps already being successfully marketed in Asia. The EcoCute
EcoCute
The EcoCute is an energy efficient electric heat pump, water heating and supply system that uses heat extracted from the air to heat water for domestic, industrial and commercial use. Instead of the more conventional ammonia or haloalkane gases, EcoCute uses supercritical carbon dioxide as a...
systems from Japan, developed by Mayekawa, develop high temperature domestic water with small inputs of electric power by moving heat into the system from their surroundings. Their success makes a future use in other world regions possible.
Supercritical carbon dioxide has been used for more than 30 years to enhance oil recovery in mature oil fields. At the same time, there is the possibility of using the various "clean coal" technologies which are emerging to combine such enhanced recovery methods with carbon sequestration efforts. Using gasifiers instead of conventional furnaces, coal and water is reduced to hydrogen gas, carbon dioxide, and ash. This hydrogen gas can be used to produce electrical power in combined-cycle gas turbines, while the CO2 is captured, compressed to the supercritical state, and injected into geological storage, possibly into existing oil fields to improve yields. The unique properties of supercritical CO2 ensure that it will remain out of the atmosphere.
It has been suggested that supercritical carbon dioxide could be used as a working fluid in Enhanced Geothermal Systems
Enhanced geothermal systems
Enhanced Geothermal System is a new type of geothermal power technology that does not require natural convective hydrothermal resources. Until recently, geothermal power systems have exploited only resources where naturally occurring heat, water, and rock permeability are sufficient to allow...
. Possible advantages compared to water include higher energy yield resulting from its lower viscosity, better chemical interaction, CO2 storage through fluid loss and higher temperature limit. As of 2011, the concept has never been tested on the field.
Sterilization of biomedical materials
Recent studies have proved SC-CO2 is an effective alternative for terminal sterilization of biological materials and medical devices. Moreover, this process is gentle, as the morphology, ultrastructure, and protein profiles of inactivated microbes are maintained.See also
- Alliance for CO2 Solutions
- CaffeineCaffeineCaffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug. Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants...
- Dry cleaningDry cleaningDry cleaning is any cleaning process for clothing and textiles using a chemical solvent other than water. The solvent used is typically tetrachloroethylene , abbreviated "perc" in the industry and "dry-cleaning fluid" by the public...
- PerfumePerfumePerfume is a mixture of fragrant essential oils and/or aroma compounds, fixatives, and solvents used to give the human body, animals, objects, and living spaces "a pleasant scent"...
- Supercritical fluidSupercritical fluidA supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
- The Cool WarThe Cool WarThe Cool War refers to the debate about the next-generation refrigerant in car air conditioning worldwide, with an ongoing dispute between the Alliance for CO2 Solutions supporting the uptake of sustainable carbon dioxide as a refrigerant in passenger cars, and chemical giants developing new...

