Sulfur tetrafluoride
Encyclopedia
Sulfur tetrafluoride is the chemical compound
with the formula S
F4
. This species exists as a gas at standard conditions. It is a corrosive species that releases dangerous HF
upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent
for the preparation of organofluorine compounds
, some of which are important in the pharmaceutical and specialty chemical industries.
. Of sulfur's total of six valence electron
s, two form a lone pair
. The structure of SF4 can therefore be anticipated using the principles of VSEPR theory
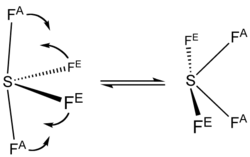
: it is a see-saw shape, with S at the center. One of the three equatorial positions is occupied by a nonbonding lone pair
of electrons. Consequently, the molecule has two distinct types of F ligands, two axial
and two equatorial
. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. In contrast to SF4, the related molecule SF6 has sulfur in the 6+ state, no valence electrons remain nonbonding on sulfur, hence the molecule adopts a highly symmetrical octahedral structure. Further contrasting with SF4, SF6 is extraordinarily inert chemically.
The 19F NMR
spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation
.
, Cl2
, and NaF
:
Treatment of SCl2 with NaF also affords SF4, not SF2. SF2 is unstable, it condenses with itself to form SF4 and SSF2.
, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. Certain alcohol
s readily give the corresponding fluorocarbon
. Ketones and aldehydes give geminal difluorides. The presence of protons alpha to the carbonyl leads to side reactions and diminished (30–40%) yield. Also diols can give cyclic sulfite esters, (RO)2SO. Carboxylic acids convert to trifluoromethyl derivatives. For example treatment of heptanoic acid with SF4 at 100-130 °C produces 1,1,1-trifluoroheptane. The coproducts from these fluorinations, including unreacted SF4 together with SOF2 and SO2, are toxic but can be neutralized by their treatment with aqueous KOH.
The use of SF4 is being superseded in recent years by the more conveniently handled diethylaminosulfur trifluoride
, Et2NSF3, "DAST", where Et = CH3CH2. This reagent is prepared from SF4:
, a useful source of the SF5 group, is prepared from SF4.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula S
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
F4
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
. This species exists as a gas at standard conditions. It is a corrosive species that releases dangerous HF
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
for the preparation of organofluorine compounds
Fluorocarbon
Fluorocarbons, sometimes referred to as perfluorocarbons or PFCs, are organofluorine compounds that contain only carbon and fluorine bonded together in strong carbon–fluorine bonds. Fluoroalkanes that contain only single bonds are more chemically and thermally stable than alkanes...
, some of which are important in the pharmaceutical and specialty chemical industries.
Structure
Sulfur in SF4 is in the formal +4 oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
. Of sulfur's total of six valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s, two form a lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
. The structure of SF4 can therefore be anticipated using the principles of VSEPR theory
VSEPR theory
Valence shell electron pair repulsion theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion. It is also named Gillespie–Nyholm theory after its two main developers...
: it is a see-saw shape, with S at the center. One of the three equatorial positions is occupied by a nonbonding lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
of electrons. Consequently, the molecule has two distinct types of F ligands, two axial
Axial
Axial may mean:* Along the same line as an axis of rotation in geometry* A type of modal frame in music* One of several anatomical directions in an animal body* Axial age, the period from 800 to 200 BC in China, India and the western world...
and two equatorial
Equatorial
Equatorial may refer to:* Equator of the Earth* Equatorial climate in meteorology* The ring-shaped outer boundary of the cross-section of a round three dimensional shape or object in geometry* The equatorial bond of a molecule in chemistry...
. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. In contrast to SF4, the related molecule SF6 has sulfur in the 6+ state, no valence electrons remain nonbonding on sulfur, hence the molecule adopts a highly symmetrical octahedral structure. Further contrasting with SF4, SF6 is extraordinarily inert chemically.
The 19F NMR
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation
Pseudorotation
The IUPAC defines pseudorotation as "a conformational change resulting in a structure that appears to have been produced by rotation of the entire initial molecule and is superimposable on the initial one, unless different positions are distinguished by substitution or isotopic labeling...
.

Synthesis and manufacture
SF4 is produced by the reaction of SCl2Sulfur dichloride
Sulfur dichloride is the chemical compound with the formula SCl2. This cherry-red liquid is the simplest sulfur chloride and one of the most common. It is used as a precursor to organosulfur compounds.-Chlorination of sulfur:...
, Cl2
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, and NaF
Sodium fluoride
Sodium fluoride is an inorganic chemical compound with the formula NaF. A colorless solid, it is a source of the fluoride ion in diverse applications. Sodium fluoride is less expensive and less hygroscopic than the related salt potassium fluoride....
:
- SCl2 + Cl2 + 4 NaF → SF4 + 4 NaCl
Treatment of SCl2 with NaF also affords SF4, not SF2. SF2 is unstable, it condenses with itself to form SF4 and SSF2.
Use of SF4 for the synthesis of fluorocarbons
In organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. Certain alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s readily give the corresponding fluorocarbon
Fluorocarbon
Fluorocarbons, sometimes referred to as perfluorocarbons or PFCs, are organofluorine compounds that contain only carbon and fluorine bonded together in strong carbon–fluorine bonds. Fluoroalkanes that contain only single bonds are more chemically and thermally stable than alkanes...
. Ketones and aldehydes give geminal difluorides. The presence of protons alpha to the carbonyl leads to side reactions and diminished (30–40%) yield. Also diols can give cyclic sulfite esters, (RO)2SO. Carboxylic acids convert to trifluoromethyl derivatives. For example treatment of heptanoic acid with SF4 at 100-130 °C produces 1,1,1-trifluoroheptane. The coproducts from these fluorinations, including unreacted SF4 together with SOF2 and SO2, are toxic but can be neutralized by their treatment with aqueous KOH.
The use of SF4 is being superseded in recent years by the more conveniently handled diethylaminosulfur trifluoride
Diethylaminosulfur trifluoride
Diethylaminosulfur trifluoride is the organosulfur compound with the formula Et2NSF3. This liquid is a fluorinating reagent used for the synthesis of organofluorine compounds...
, Et2NSF3, "DAST", where Et = CH3CH2. This reagent is prepared from SF4:
- SF4 + Me3SiNEt2 → Et2NSF3 + Me3SiF
Other reactions
Sulfur chloride pentafluorideSulfur chloride pentafluoride
Sulfur chloride pentafluoride is an inorganic compound with the formula . It exists as a colorless gas at room temperature and is highly toxic. The compound has an octahedral geometry with symmetry...
, a useful source of the SF5 group, is prepared from SF4.

