Alkyl nitrites
Encyclopedia
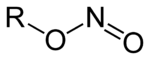
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s based upon the molecular structure R-ONO. Formally they are alkyl ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s of nitrous acid
Nitrous acid
Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
. They are distinct from nitro compound
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
s (R-NO2).
The first few members of the series are volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
liquids; methyl nitrite
Methyl nitrite
In organic chemistry, methyl nitrite is the simplest alkyl nitrite.-Structure:At room temperature, methyl nitrite exists as a mixture of cis and trans conformers...
and ethyl nitrite
Ethyl nitrite
The chemical compound ethyl nitrite is an alkyl nitrite. It may be prepared from ethanol.Ethyl nitrite is the main ingredient in a traditional ethanol-based South African remedy for colds and flu known as Witdulsies and sold in pharmacies...
are gaseous at room temperature and pressure. The compounds have a distinctive fruity odor. Another frequently encountered nitrite is amyl nitrite
Amyl nitrite
Amyl nitrite is the chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrito functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group...
(3-methylbutyl nitrite).
Alkyl nitrites were initially, and largely still are used as medication
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
s and chemical reagents, a practice which began in the late 19th century. In their use as medicine, nitrite vapors are often inhaled for relief of angina
Angina
Angina pectoris, commonly known as angina, is chest pain due to ischemia of the heart muscle, generally due to obstruction or spasm of the coronary arteries . Coronary artery disease, the main cause of angina, is due to atherosclerosis of the cardiac arteries...
and other heart-related symptoms of disease. However when referred to as "poppers
Poppers
Poppers is a slang term for various alkyl nitrites inhaled for recreational purposes, particularly isopropyl nitrite and isobutyl nitrite , and now more rarely, butyl nitrite and amyl nitrite...
", alkyl nitrites represent recreational drugs.
Synthesis
Organic nitrites are prepared from alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s and sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
in sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
. They decompose slowly on standing, the decomposition products being oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, the alcohol, and polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
products of the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
.
Reactions
- In the laboratory, solutions of alkyl nitrites in glacial acetic acid are sometimes used as mild nitrating agentsNitrationNitration is a general chemical process for the introduction of a nitro group into a chemical compound. The dominant application of nitration is for the production of nitrobenzene, the precursor to methylene diphenyl diisocyanate...
. The nitrating species formed is acetyl nitrate generated in situIn situIn situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
. - n-Butyl nitrite and ammoniaAmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
convert phenylhydroxylaminePhenylhydroxylaminePhenylhydroxylamine is the organic compound with the formula C6H5NHOH. It is an intermediate in the redox-related pair C6H5NH2 and C6H5NO. Phenylhydroxylamine should not be confused with its isomer α-phenylhydroxylamine or O-phenylhydroxylamine, is C6H5ONH2.-Preparation and derivatives:This...
to its nitrosamineNitrosamineNitrosamines are chemical compounds of the chemical structure R1N-N=O, some of which are carcinogenic.-Usages:Nitrosamines are used in manufacture of some cosmetics, pesticides, and in most rubber products. -Occurrences:...
derivative cupferronCupferronCupferron, the ammonium salt of N-nitroso-N-phenylhydroxylamine, is a common reagent for the complexation of metal ions. Its formula is...
. Likewise pyrrolidinePyrrolidinePyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula C4H9N. It is a cyclic secondary amine with a five-membered heterocycle containing four carbon atoms and one nitrogen atom...
is a substrate for ethyl nitrite. - Alkyl nitrites are also used in the formation of oximeOximeAn oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s with the stronger carbon acids and acid or base catalysis for example in the reaction of 2-butanone, ethyl nitriteEthyl nitriteThe chemical compound ethyl nitrite is an alkyl nitrite. It may be prepared from ethanol.Ethyl nitrite is the main ingredient in a traditional ethanol-based South African remedy for colds and flu known as Witdulsies and sold in pharmacies...
and hydrochloric acidHydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
forming the oxime, the similar reaction with phenacyl chloride, or the reaction of phenylacetonitrile with methyl nitriteMethyl nitriteIn organic chemistry, methyl nitrite is the simplest alkyl nitrite.-Structure:At room temperature, methyl nitrite exists as a mixture of cis and trans conformers...
and sodium hydroxide.
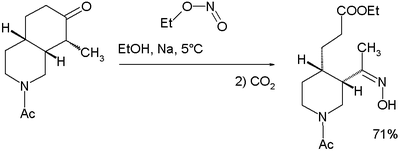
An isolated but classic example of the use of alkyl nitrites can be found in Woodward and Doering's quinine total synthesis
Quinine total synthesis
In total synthesis, the Quinine total synthesis describes the efforts in synthesis of quinine over a 150 year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine...
:
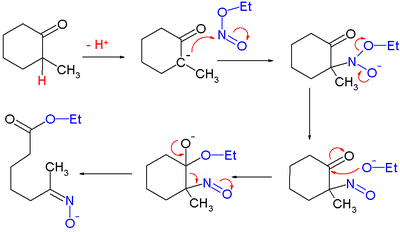
for which they proposed this reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
: