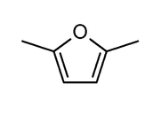
2,5-Dimethylfuran
Encyclopedia
2,5-Dimethylfuran is a heterocyclic compound
with the formula
(CH3)2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide
. A derivative of furan
, this simple compound is a potential biofuel
, being derivable from cellulose.
can be converted into 2,5-dimethylfuran in a catalytic
biomass-to-liquid process. The conversion of fructose to DMF proceeds via hydroxymethylfurfural
.
Fructose is obtainable from glucose, a building block in cellulose.
. It has an energy density
40% greater than ethanol
, making it comparable to gasoline
(petrol). It is also chemically stable and, being insoluble in water, does not absorb moisture from the atmosphere. Evaporating
dimethylfuran during the production process also requires around one third less energy than the evaporation of ethanol, although it has a boiling point some 14 °C higher, at 92 °C, compared to 78 °C for ethanol.
The ability to efficiently and rapidly produce dimethylfuran from fructose, found in fruit
and some root vegetable
s, or from glucose, which can be derived from starch
and cellulose
- all widely available in nature - adds to the attraction of dimethylfuran, although safety issues must be examined. Bioethanol and biodiesel
are currently the leading liquid biofuels.
The stoichiometric air/fuel ratio of dimethylfuran is 10.72, compared to ethanol at 8.95 and gasoline at 14.56. This means that burning dimethylfuran requires approximately 33% less air than the same quantity of gasoline, but approximately 20% more air than the same quantity of ethanol.
The calorific value of liquid dimethylfuran is 33.7 MJ/kg, compared to 26.9 MJ/kg for ethanol and 43.2 MJ/kg for gasoline. The research octane number (RON) of dimethylfuran is 119. The latent heat of vaporization at 20°C is 31.91 kJ/mol. Recent tests in a single-cylinder gasoline engine found that the thermal efficiency of burning dimethylfuran is similar to that of gasoline.
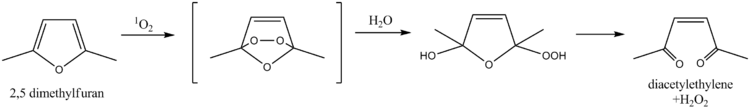
for singlet oxygen
, a property which has been exploited for the determination of singlet oxygen in natural waters. The mechanism involves a Diels-Alder reaction
followed by hydrolysis
, ultimately leading to diacetylethylene and hydrogen peroxide
as products. More recently, furfuryl alcohol
has been used for the same purpose.
This compound has also been proposed as an internal standard
for NMR spectroscopy
. 2,5-Dimethylfuran has singlets in its 1H NMR spectrum at δ 2.2 and 5.8; the singlets give reliable integrations, while the positions of the peaks do not interfere with many analytes. The compound also has an appropriate boiling point of 92 °C which prevents evaporative losses, yet is easily removed.
sugars.
of hexane in humans. Together with hexane-2,5-dione
and 4,5-dihydroxy-2-hexanone, it is one of the main metabolite
s of hexane
.
2,5-Dimethylfuran has been identified as one of the components of cigar
smoke
with low cilatoxicity (ability to adversely affect the cilia in the respiratory tract
that are responsible for removing foreign particles). Its blood concentration can be used as a biomarker for smoking
.
Comparison of MSDS sheets shows that human handling of 2,5-dimethylfuranhttps://fscimage.fishersci.com/msds/03657.htmhttp://www.chemexper.com/cheminfo/servlet/org.dbcreator.MainServlet?query=msds._msdsID%3D11090&target=msds&action=PowerSearch&history=off&format=google2008http://www.pennakem.com/msds/2.5dimethylfuran.pdf is approximately as hazardous as handling gasoline.http://www.equivashellmsds.com/getsinglemsds.asp?ID=202020http://hazard.com/msds/f2/bxw/bxwzb.htmlhttp://hazard.com/msds/f2/bhh/bhhfm.html
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
(CH3)2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
. A derivative of furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
, this simple compound is a potential biofuel
Biofuel
Biofuel is a type of fuel whose energy is derived from biological carbon fixation. Biofuels include fuels derived from biomass conversion, as well as solid biomass, liquid fuels and various biogases...
, being derivable from cellulose.
Production
FructoseFructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
can be converted into 2,5-dimethylfuran in a catalytic
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
biomass-to-liquid process. The conversion of fructose to DMF proceeds via hydroxymethylfurfural
Hydroxymethylfurfural
Hydroxymethylfurfural , also 5-furfural, is an organic compound derived from dehydration of certain sugars. This yellow low-melting solid is highly water-soluble. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups...
.
Fructose is obtainable from glucose, a building block in cellulose.
Potential as a biofuel
DMF has a number of attractions as a biofuelBiofuel
Biofuel is a type of fuel whose energy is derived from biological carbon fixation. Biofuels include fuels derived from biomass conversion, as well as solid biomass, liquid fuels and various biogases...
. It has an energy density
Energy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
40% greater than ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, making it comparable to gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
(petrol). It is also chemically stable and, being insoluble in water, does not absorb moisture from the atmosphere. Evaporating
Evaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
dimethylfuran during the production process also requires around one third less energy than the evaporation of ethanol, although it has a boiling point some 14 °C higher, at 92 °C, compared to 78 °C for ethanol.
The ability to efficiently and rapidly produce dimethylfuran from fructose, found in fruit
Fruit
In broad terms, a fruit is a structure of a plant that contains its seeds.The term has different meanings dependent on context. In non-technical usage, such as food preparation, fruit normally means the fleshy seed-associated structures of certain plants that are sweet and edible in the raw state,...
and some root vegetable
Root vegetable
Root vegetables are plant roots used as vegetables. Here "root" means any underground part of a plant.Root vegetables are generally storage organs, enlarged to store energy in the form of carbohydrates. They differ in the concentration and the balance between sugars, starches, and other types of...
s, or from glucose, which can be derived from starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
and cellulose
Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units....
- all widely available in nature - adds to the attraction of dimethylfuran, although safety issues must be examined. Bioethanol and biodiesel
Biodiesel
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl esters. Biodiesel is typically made by chemically reacting lipids with an alcohol....
are currently the leading liquid biofuels.
The stoichiometric air/fuel ratio of dimethylfuran is 10.72, compared to ethanol at 8.95 and gasoline at 14.56. This means that burning dimethylfuran requires approximately 33% less air than the same quantity of gasoline, but approximately 20% more air than the same quantity of ethanol.
The calorific value of liquid dimethylfuran is 33.7 MJ/kg, compared to 26.9 MJ/kg for ethanol and 43.2 MJ/kg for gasoline. The research octane number (RON) of dimethylfuran is 119. The latent heat of vaporization at 20°C is 31.91 kJ/mol. Recent tests in a single-cylinder gasoline engine found that the thermal efficiency of burning dimethylfuran is similar to that of gasoline.
Other uses
2,5-Dimethylfuran serves as a scavengerScavenger (chemistry)
A scavenger in chemistry is a chemical substance added to a mixture in order to remove or inactivate impurities or unwanted reaction products. Their use is wide-ranged:...
for singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
, a property which has been exploited for the determination of singlet oxygen in natural waters. The mechanism involves a Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
followed by hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
, ultimately leading to diacetylethylene and hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
as products. More recently, furfuryl alcohol
Furfuryl alcohol
Furfuryl alcohol, also called 2-furylmethanol or 2-furancarbinol, is an organic compound containing a furan substituted with a hydroxymethyl group. It is a clear colorless liquid when pure, but becomes amber colored upon prolonged standing. It possesses a faint burning odor and a bitter taste. ...
has been used for the same purpose.

This compound has also been proposed as an internal standard
Internal standard
An internal standard in analytical chemistry is a chemical substance that is added in a constant amount to samples, the blank and calibration standards in a chemical analysis. This substance can then be used for calibration by plotting the ratio of the analyte signal to the internal standard signal...
for NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
. 2,5-Dimethylfuran has singlets in its 1H NMR spectrum at δ 2.2 and 5.8; the singlets give reliable integrations, while the positions of the peaks do not interfere with many analytes. The compound also has an appropriate boiling point of 92 °C which prevents evaporative losses, yet is easily removed.
Role in food chemistry
2,5-Dimethylfuran forms upon thermal degradation of some sugars and has been identified in trace amounts as a component of caramelizedCaramelization
Caramelization is the browning of sugar, a process used extensively in cooking for the resulting nutty flavor and brown color. As the process occurs, volatile chemicals are released, producing the characteristic caramel flavor....
sugars.
Toxicology
2,5-Dimethylfuran plays a role in the mechanism for the neurotoxicityNeurotoxicity
Neurotoxicity occurs when the exposure to natural or artificial toxic substances, which are called neurotoxins, alters the normal activity of the nervous system in such a way as to cause damage to nervous tissue. This can eventually disrupt or even kill neurons, key cells that transmit and process...
of hexane in humans. Together with hexane-2,5-dione
Hexane-2,5-dione
Hexane-2,5-dione, C6H10O2, is a diketone and a toxic metabolite of hexane.-Symptoms:The initial symptoms of chronic hexane toxicity, attributable to hexane-2,5-dione, are tingling and cramps in the arms and legs, followed by general muscular weakness...
and 4,5-dihydroxy-2-hexanone, it is one of the main metabolite
Metabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
s of hexane
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
.
2,5-Dimethylfuran has been identified as one of the components of cigar
Cigar
A cigar is a tightly-rolled bundle of dried and fermented tobacco that is ignited so that its smoke may be drawn into the mouth. Cigar tobacco is grown in significant quantities in Brazil, Cameroon, Cuba, the Dominican Republic, Honduras, Indonesia, Mexico, Nicaragua, Philippines, and the Eastern...
smoke
Smoke
Smoke is a collection of airborne solid and liquid particulates and gases emitted when a material undergoes combustion or pyrolysis, together with the quantity of air that is entrained or otherwise mixed into the mass. It is commonly an unwanted by-product of fires , but may also be used for pest...
with low cilatoxicity (ability to adversely affect the cilia in the respiratory tract
Respiratory tract
In humans the respiratory tract is the part of the anatomy involved with the process of respiration.The respiratory tract is divided into 3 segments:*Upper respiratory tract: nose and nasal passages, paranasal sinuses, and throat or pharynx...
that are responsible for removing foreign particles). Its blood concentration can be used as a biomarker for smoking
Smoking
Smoking is a practice in which a substance, most commonly tobacco or cannabis, is burned and the smoke is tasted or inhaled. This is primarily practised as a route of administration for recreational drug use, as combustion releases the active substances in drugs such as nicotine and makes them...
.
Comparison of MSDS sheets shows that human handling of 2,5-dimethylfuranhttps://fscimage.fishersci.com/msds/03657.htmhttp://www.chemexper.com/cheminfo/servlet/org.dbcreator.MainServlet?query=msds._msdsID%3D11090&target=msds&action=PowerSearch&history=off&format=google2008http://www.pennakem.com/msds/2.5dimethylfuran.pdf is approximately as hazardous as handling gasoline.http://www.equivashellmsds.com/getsinglemsds.asp?ID=202020http://hazard.com/msds/f2/bxw/bxwzb.htmlhttp://hazard.com/msds/f2/bhh/bhhfm.html

