Hydroxymethylfurfural
Encyclopedia
Hydroxymethylfurfural also 5-(Hydroxymethyl)furfural, is an organic compound
derived from dehydration of certain sugars. This yellow low-melting solid is highly water-soluble. The molecule consists of a furan
ring, containing both aldehyde
and alcohol
functional groups. HMF has been identified in a wide variety of heat-processed foods including milk
, fruit juices, spirits, honey
, etc. HMF, which is derived from cellulose
without use of fermentation
, is a potential "carbon-neutral" feedstock for fuels and chemicals.
, HMF is produced from sugars. It arises via the dehydration of fructose
. Treatment of fructose with acids followed by liquid-liquid extraction
into organic solvents such as methyl isobutyl ketone
. The conversion is affected by various additives such as DMSO
, 2-butanol
, and Poly vinyl pyrrolidone
, which minimize the formation of side product. In an optimized system for fructose (but not raw biomass), conversion is 77%, with half the HMF ending up in the organic phase. Ionic liquids also facilitate the conversion of fructose to HMF.
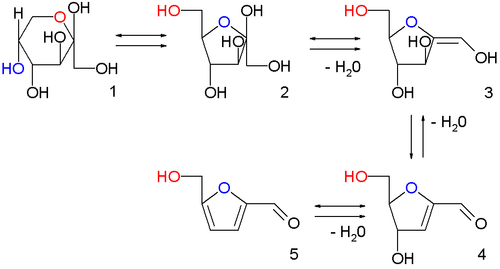
In the image above are displayed in a series of chemical equilibria
: fructopyranose 1, fructofuranose 2, two intermediate stages of dehydration
(not isolated) 3,4 and finally HMF 5.
Chromous chloride catalyzes the direct conversion of both fructose
(yielding 90%+) and glucose
(yielding 70%+) into an HMF.
Subsequently cellulose has been directly converted into HMF (yielding 55% at 96% purity). The chromium chloride catalyzes the conversion of glucose into fructose.
(DMF), which is a liquid biofuel that in certain ways is superior to ethanol. Oxidation of HMF also gives 2,5-furandicarboxylic acid
, which has been proposed as a replacement for terephthalic acid
in the production of polyesters.
5-Hydroxymethyl-2-furfural (5HMF) has been found to bind specifically with intracellular sickle hemoglobin (HbS). Preliminary in vivo studies using transgenic sickle mice showed that orally administered 5HMF inhibits the formation of sickled cells in the blood.
as well as during caramelization
. In these foods it is also slowly generated during storage. Acid conditions favour generation of HMF.
HMF can be found in low amounts in honey
, fruit-juice
s and UHT-milk. Here as well as in vinegars, jams, alcoholic products or biscuits HMF can be used as an indicator for excess heat-treatment. For instance, fresh honey only has low amounts of HMF—less than 15 mg/kg—depending on pH-value and temperature and age, and the codex alimentarius standard requires that honey have less than 40 mg/kg HMf to guarantee that the honey has not undergone heating during processing, except for tropical honeys which must be below 80 mg/kg.
Higher quantities of HMF are found naturally in coffee and dried fruit. Several types of roasted coffee contained between 300 – 2900 mg/kg HMF. Dried plums were found to contain up to 2200 mg/kg HMF. In dark beer 13.3 mg/kg were found, bakery-products contained between 4.1 – 151 mg/kg HMF.
HMF can form in high-fructose corn syrup (HFCS), levels around 20 mg/kg HMF were found, increasing during storage or heating. This is a problem for American beekeepers because they use HFCS as a source of sugar when there are not enough nectar sources to feed honeybees, and HMF is toxic to them. Adding bases such as soda ash or potash to neutralize
the HFCS slows down the formation of HMF.
Depending on production-technology and storage, levels in food vary considerably. To evaluate the contribution of a food to HMF intake, its consumption-pattern has to be considered. Coffee is the food that has a very high relevance in terms of levels of HMF and quantities consumed.
HMF is a natural component in heated food but usually present in low concentrations. The daily intake of HMF may underlie high variations due to individual consumption-patterns. It has been estimated that in a western diet, in the order of magnitude of 5 – 10 mg of HMF are ingested per day from food.
In former times, HMF was used in food for flavoring purposes, but in Europe this practice now is suspended. HMF is also found in cigarette smoke.
in humans is 5-hydroxymethyl-2-furoic acid (HMFA), which is excreted in urine. HMF can also be metabolized to 5-sulfoxymethylfurfural (SMF), which is highly reactive and can form adducts with DNA or proteins. In vitro tests and studies on rats suggest potential toxicity and carcinogenicity of HMF. In humans, no correlation of intakes of HMF and disease could be demonstrated yet.
with UV-detection is the reference-method (e.g. DIN 10751-3). Classic methods for the quantification of HMF in food use photometry
. The method according to White is a differential UV-photometry with and without sodium bisulphite-reduction of HMF (AOAC 980.23). Winkler photometric method is a colour-reaction using p-toluidine
and barbituric acid
(DIN
10751-1). Photometric test may be unspecific as they may detect also related substances, leading to higher results than HPLC-measurements. Test-kits for rapid analyses are also available (e.g. Refelctoquant HMF, Merck KGaA).
was studied by French
chemist
Louis Maillard
in 1912 in studies on non-enzymatic reactions of glucose
.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
derived from dehydration of certain sugars. This yellow low-melting solid is highly water-soluble. The molecule consists of a furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
ring, containing both aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
and alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
functional groups. HMF has been identified in a wide variety of heat-processed foods including milk
Milk
Milk is a white liquid produced by the mammary glands of mammals. It is the primary source of nutrition for young mammals before they are able to digest other types of food. Early-lactation milk contains colostrum, which carries the mother's antibodies to the baby and can reduce the risk of many...
, fruit juices, spirits, honey
Honey
Honey is a sweet food made by bees using nectar from flowers. The variety produced by honey bees is the one most commonly referred to and is the type of honey collected by beekeepers and consumed by humans...
, etc. HMF, which is derived from cellulose
Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units....
without use of fermentation
Industrial fermentation
Industrial fermentation is the intentional use of fermentation by microorganisms such as bacteria and fungi to make products useful to humans. Fermented products have applications as food as well as in general industry.- Food fermentation :...
, is a potential "carbon-neutral" feedstock for fuels and chemicals.
Production
Related to the production of furfuralFurfural
Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word , meaning bran, referring to its usual source....
, HMF is produced from sugars. It arises via the dehydration of fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
. Treatment of fructose with acids followed by liquid-liquid extraction
Liquid-liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid...
into organic solvents such as methyl isobutyl ketone
Methyl isobutyl ketone
Methyl isobutyl ketone is the organic compound with the formula 2CHCH2CCH3. This colourless liquid, a ketone, is widely used as a solvent.-Production:...
. The conversion is affected by various additives such as DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, 2-butanol
2-Butanol
2-Butanol, or sec-butanol, is an organic compound with formula CH3CHCH2CH3. This secondary alcohol is a flammable, colorless liquid that is soluble in 12 parts water and completely miscible with polar organic solvents such as ethers and other alcohols. It is produced on a large scale, primarily as...
, and Poly vinyl pyrrolidone
Poly vinyl pyrrolidone
Polyvinylpyrrolidone , also commonly called Polyvidone or Povidone, is a water-soluble polymer made from the monomer N-vinylpyrrolidone:- Properties :...
, which minimize the formation of side product. In an optimized system for fructose (but not raw biomass), conversion is 77%, with half the HMF ending up in the organic phase. Ionic liquids also facilitate the conversion of fructose to HMF.
In the image above are displayed in a series of chemical equilibria
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
: fructopyranose 1, fructofuranose 2, two intermediate stages of dehydration
Dehydration
In physiology and medicine, dehydration is defined as the excessive loss of body fluid. It is literally the removal of water from an object; however, in physiological terms, it entails a deficiency of fluid within an organism...
(not isolated) 3,4 and finally HMF 5.
Chromous chloride catalyzes the direct conversion of both fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
(yielding 90%+) and glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
(yielding 70%+) into an HMF.
Subsequently cellulose has been directly converted into HMF (yielding 55% at 96% purity). The chromium chloride catalyzes the conversion of glucose into fructose.
Uses
HMF can be converted to 2,5-dimethylfuran2,5-Dimethylfuran
2,5-Dimethylfuran is a heterocyclic compound with the formula 2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose.-Production:Fructose can be converted into...
(DMF), which is a liquid biofuel that in certain ways is superior to ethanol. Oxidation of HMF also gives 2,5-furandicarboxylic acid
2,5-Furandicarboxylic acid
2,5-Furandicarboxylic acid , also known as dehydromucic acid, is an oxidized furan derivative. This organic compound was first obtained by Fittig and Heinzelmann in 1876. The first review by Henry Hill was already published in 1901. FDCA has also been detected in human urine. A healthy human...
, which has been proposed as a replacement for terephthalic acid
Terephthalic acid
Terephthalic acid is the organic compound with formula C6H42. This colourless solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several billion kilograms are produced annually...
in the production of polyesters.
5-Hydroxymethyl-2-furfural (5HMF) has been found to bind specifically with intracellular sickle hemoglobin (HbS). Preliminary in vivo studies using transgenic sickle mice showed that orally administered 5HMF inhibits the formation of sickled cells in the blood.
As a component in food
HMF is practically not present in fresh food, but it is naturally generated in sugar-containing food during heat-treatments like drying or cooking. Along with many other flavor- and colour-related substances, HMF is formed in the Maillard reactionMaillard reaction
The Maillard reaction is a form of nonenzymatic browning similar to caramelization. It results from a chemical reaction between an amino acid and a reducing sugar, usually requiring heat....
as well as during caramelization
Caramelization
Caramelization is the browning of sugar, a process used extensively in cooking for the resulting nutty flavor and brown color. As the process occurs, volatile chemicals are released, producing the characteristic caramel flavor....
. In these foods it is also slowly generated during storage. Acid conditions favour generation of HMF.
HMF can be found in low amounts in honey
Honey
Honey is a sweet food made by bees using nectar from flowers. The variety produced by honey bees is the one most commonly referred to and is the type of honey collected by beekeepers and consumed by humans...
, fruit-juice
Juice
Juice is the liquid that is naturally contained in fruit or vegetable tissue.Juice is prepared by mechanically squeezing or macerating fruit or vegetable flesh without the application of heat or solvents. For example, orange juice is the liquid extract of the fruit of the orange tree...
s and UHT-milk. Here as well as in vinegars, jams, alcoholic products or biscuits HMF can be used as an indicator for excess heat-treatment. For instance, fresh honey only has low amounts of HMF—less than 15 mg/kg—depending on pH-value and temperature and age, and the codex alimentarius standard requires that honey have less than 40 mg/kg HMf to guarantee that the honey has not undergone heating during processing, except for tropical honeys which must be below 80 mg/kg.
Higher quantities of HMF are found naturally in coffee and dried fruit. Several types of roasted coffee contained between 300 – 2900 mg/kg HMF. Dried plums were found to contain up to 2200 mg/kg HMF. In dark beer 13.3 mg/kg were found, bakery-products contained between 4.1 – 151 mg/kg HMF.
HMF can form in high-fructose corn syrup (HFCS), levels around 20 mg/kg HMF were found, increasing during storage or heating. This is a problem for American beekeepers because they use HFCS as a source of sugar when there are not enough nectar sources to feed honeybees, and HMF is toxic to them. Adding bases such as soda ash or potash to neutralize
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
the HFCS slows down the formation of HMF.
Depending on production-technology and storage, levels in food vary considerably. To evaluate the contribution of a food to HMF intake, its consumption-pattern has to be considered. Coffee is the food that has a very high relevance in terms of levels of HMF and quantities consumed.
HMF is a natural component in heated food but usually present in low concentrations. The daily intake of HMF may underlie high variations due to individual consumption-patterns. It has been estimated that in a western diet, in the order of magnitude of 5 – 10 mg of HMF are ingested per day from food.
In former times, HMF was used in food for flavoring purposes, but in Europe this practice now is suspended. HMF is also found in cigarette smoke.
Metabolism
A major metaboliteMetabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
in humans is 5-hydroxymethyl-2-furoic acid (HMFA), which is excreted in urine. HMF can also be metabolized to 5-sulfoxymethylfurfural (SMF), which is highly reactive and can form adducts with DNA or proteins. In vitro tests and studies on rats suggest potential toxicity and carcinogenicity of HMF. In humans, no correlation of intakes of HMF and disease could be demonstrated yet.
Quantification
Today, HPLCHigh-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
with UV-detection is the reference-method (e.g. DIN 10751-3). Classic methods for the quantification of HMF in food use photometry
Photometry
Photometry can refer to:* Photometry , the science of measurement of visible light in terms of its perceived brightness to human vision...
. The method according to White is a differential UV-photometry with and without sodium bisulphite-reduction of HMF (AOAC 980.23). Winkler photometric method is a colour-reaction using p-toluidine
Toluidine
There are three isomers of toluidine, which are organic compounds. These isomers are o-toluidine, m-toluidine, and p-toluidine. The o- stands for ortho- , m- stands for meta- , and p- stands for para-...
and barbituric acid
Barbituric acid
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active...
(DIN
Din
DIN or Din or din can have several meanings:* A din is a loud noise.* Dīn, an Arabic term meaning "religion" or "way of life".* Din is one of the ten aspects of the Ein Sof in Kabbalah ....
10751-1). Photometric test may be unspecific as they may detect also related substances, leading to higher results than HPLC-measurements. Test-kits for rapid analyses are also available (e.g. Refelctoquant HMF, Merck KGaA).
History
This organic compoundOrganic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
was studied by French
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Louis Maillard
Louis Camille Maillard
Louis Camille Maillard was a French physician and chemist.-Early days:He was admitted to the Faculty of Science in the University of Nancy at the age of 16...
in 1912 in studies on non-enzymatic reactions of glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
.