
Aflatoxin total synthesis
Encyclopedia
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of a group of organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s called aflatoxin
Aflatoxin
Aflatoxins are naturally occurring mycotoxins that are produced by many species of Aspergillus, a fungus, the most notable ones being Aspergillus flavus and Aspergillus parasiticus. Aflatoxins are toxic and among the most carcinogenic substances known...
s. These compounds occur naturally in several fungi. As with other chemical compound targets in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, the organic synthesis of aflatoxins serve different purposes. Traditionally it served to prove the structure of a complex biocompound in addition to evidence obtained from spectroscopy. It also demonstrates new concepts in organic chemistry (reagents, reaction types) and it also opens the way to molecular derivatives not found in nature. Last but not least a synthetic biocompound is a commercial alternative to isolating the compound from natural resources. Aflatoxins in particular add another dimension because it is suspected that they have been mass produced in the past from biological sources as part of a biological weapons program.
The synthesis of racemic aflatoxin B1 has been reported by Buechi et al. in 1967 and that of racemic aflatoxin B2 by Roberts et al. in 1968 The group of Barry Trost
Barry Trost
Barry M. Trost is an American chemist, Tamaki Professor of Humanities and Sciences at Stanford University....
of Stanford University
Stanford University
The Leland Stanford Junior University, commonly referred to as Stanford University or Stanford, is a private research university on an campus located near Palo Alto, California. It is situated in the northwestern Santa Clara Valley on the San Francisco Peninsula, approximately northwest of San...
is responsible for the stereoselective total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of (+)-Aflatoxin B1 and B2a in 2003. In 2005 the group of E. J. Corey
Elias James Corey
Elias James Corey is an American organic chemist. In 1990 he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis...
of Harvard University
Harvard University
Harvard University is a private Ivy League university located in Cambridge, Massachusetts, United States, established in 1636 by the Massachusetts legislature. Harvard is the oldest institution of higher learning in the United States and the first corporation chartered in the country...
presented the enantioselective synthesis of Aflatoxin B2.
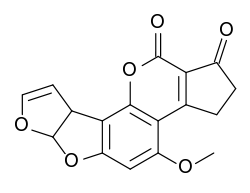
Aflatoxin B2 synthesis
The total synthesis of Aflatoxin B2 is a multistep sequence that begins with a [2+3]cycloadditionCycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
between the quinone
Quinone
A quinone is a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C– groups with any necessary rearrangement of double bonds," resulting in "a fully conjugated cyclic dione structure."...
1 and the 2,3-Dihydrofuran
2,3-Dihydrofuran
2,3-Dihydrofuran is a heterocyclic compound. It is one of the simplest enol ethers....
. This reaction is catalyzed by a CBS catalyst
CBS catalyst
The CBS catalyst or Corey-Bakshi-Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and [3+2] cycloadditions. Proline, a naturally occurring chiral compound, is readily and cheaply available...
and is enantioselective. The next step is the orthoformylation
Formylation reaction
A formylation reaction in organic chemistry is the catch-all name for any organic reaction in which an organic compound is functionalized with a formyl group .Aromatic formylation reactions via electrophilic aromatic substitution include:...
of reaction product 2 in a Duff reaction
Duff reaction
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source...
. The hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group in 3 is esterified with triflic anhydride
Triflic anhydride
Triflic anhydride is the chemical compound with the formula 2O. This compound is a particularly strong electrophile, useful for introducing the triflyl group, CF3SO2. Triflic anhydride is the acid anhydride of the strong acid triflic acid, CF3SO2OH....
which adds a triflate protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
. This step enables a Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
of the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
group in 4 with methylmagnesiumbromide to the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
5 which is then oxidized with the Dess-Martin periodinane
Dess-Martin periodinane
Dess–Martin periodinane is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions , shorter reaction times, higher yields, simplified workups,...
to the ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
6. A Baeyer-Villiger oxidation
Baeyer-Villiger oxidation
The Baeyer–Villiger oxidation is an organic reaction in which a ketone is oxidized to an ester by treatment with peroxy acids or hydrogen peroxide. Key features of the Baeyer–Villiger oxidation are its stereospecificity and predictable regiochemistry...
converts the ketone to an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
(7) and a reduction with Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
converts the ester into an alcohol and removes the triflic acid group. In the final step the coumarin
Coumarin
Coumarin is a fragrant chemical compound in the benzopyrone chemical class, found in many plants, notably in high concentration in the tonka bean , vanilla grass , sweet woodruff , mullein , sweet grass , cassia cinnamon and sweet clover...
skeleton is added to 9 by a combined coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
with zinc carbonate of the vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
bromide in 8 and a transesterification step between the phenol group and the ethyl ester group.
| Aflatoxin B2 total synthesis |
|---|

