Algar-Flynn-Oyamada reaction
Encyclopedia
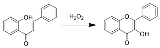
The Algar–Flynn–Oyamada reaction is a chemical reaction
whereby a chalcone
undergoes an oxidative
cyclization to form a flavonol.
One point we should notice is that those mechanisms which have a epoxide
to be an intermediate are excluded,which should be obtained by the oxidation of the double bond with hydrogen peroxide in Prileschajew reaction. Gormley et al. have shown that the reaction does not proceed through an epoxide..
The probable mechanisms are thus two possibilities:
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
whereby a chalcone
Chalconoid
Chalconoids, also known as chalcones, are natural phenols related to chalcone. They form the central core for a variety of important biological compounds. They show antibacterial, antifungal, antitumor and anti-inflammatory properties...
undergoes an oxidative
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
cyclization to form a flavonol.
Reaction mechanism
There are several possible mechanisms to explain this reaction, however, these reaction mechanisms have not been elucidated. What is known is that a two-stage mechanism exists. First, dihydroflavonol is formed, which then subsequently oxidized to form Flavonol.One point we should notice is that those mechanisms which have a epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
to be an intermediate are excluded,which should be obtained by the oxidation of the double bond with hydrogen peroxide in Prileschajew reaction. Gormley et al. have shown that the reaction does not proceed through an epoxide..
The probable mechanisms are thus two possibilities:
- The attack of nucleophiles by Base phenolates educated at the double bond under direct attack on the hydrogen peroxide.
- Nucleophiles attack phenolates under the formation of a enolate, which then attacks on hydrogen peroxide.
See also
- Allan-Robinson reactionAllan-Robinson reactionThe Allan-Robinson reaction is the chemical reaction of o-hydroxyaryl ketones with aromatic anhydrides to form flavones .Note that if aliphatic anhydrides are used, coumarins can also be formed. -Mechanism:...
- Auwers synthesisAuwers synthesisThe Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone. This reaction was first reported by Karl von Auwers in 1908....

