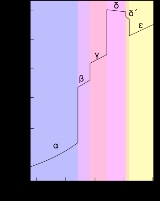
Allotropes of plutonium
Encyclopedia

Plutonium normally has six allotropes and forms a seventh (zeta, ζ) under high temperature and a limited pressure range. These allotropes have very similar energy level
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
s but significantly varying densities
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
and crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
s. This makes plutonium very sensitive to changes in temperature, pressure, or chemistry, and allows for dramatic volume changes following phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
s. Unlike most materials, plutonium increases in density when it melts, by 2.5%, but the liquid metal exhibits a linear decrease in density with temperature. Densities of the different allotropes vary from 16.00 g/cm3 to 19.86 g/cm3.
The presence of these many allotropes makes machining plutonium very difficult, as it changes state very readily. For example, the α phase exists at room temperature in unalloyed plutonium. It has machining characteristics similar to cast iron
Cast iron
Cast iron is derived from pig iron, and while it usually refers to gray iron, it also identifies a large group of ferrous alloys which solidify with a eutectic. The color of a fractured surface can be used to identify an alloy. White cast iron is named after its white surface when fractured, due...
but changes to the plastic and easy to work β phase (beta phase) at slightly higher temperatures. The reasons for the complicated phase diagram are not entirely understood; recent research has focused on constructing accurate computer models of the phase transitions. The α phase has a low-symmetry monoclinic
Monoclinic crystal system
In crystallography, the monoclinic crystal system is one of the 7 lattice point groups. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. They form a rectangular prism with a...
structure, hence its poor conductivity, brittleness, strength and compressibility.
Plutonium in the δ phase (delta phase) normally exists in the 310 °C to 452 °C range but is stable at room temperature when alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
ed with a small percentage of gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
, aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, or cerium
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
, enhancing workability and allowing it to be welded
Welding
Welding is a fabrication or sculptural process that joins materials, usually metals or thermoplastics, by causing coalescence. This is often done by melting the workpieces and adding a filler material to form a pool of molten material that cools to become a strong joint, with pressure sometimes...
in weapons applications. The delta phase has more typical metallic character, and is roughly as strong and malleable as aluminium. In fission weapons, the explosive shock wave
Shock wave
A shock wave is a type of propagating disturbance. Like an ordinary wave, it carries energy and can propagate through a medium or in some cases in the absence of a material medium, through a field such as the electromagnetic field...
s used to compress a plutonium core will also cause a transition from the usual delta phase plutonium to the denser alpha phase, significantly helping to achieve supercriticality. The plutonium-gallium alloy
Plutonium-gallium alloy
Plutonium-gallium alloy is an alloy of plutonium and gallium, used in nuclear weapon pits – the component of a nuclear weapon where the fission chain reaction is started....
is the most common δ-stabilized alloy.
Gallium
Gallium
Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies...
, aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, americium
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
, scandium
Scandium
Scandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic transition metal, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids...
and cerium
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
can stabilize the δ phase of plutonium for room temperature. Silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, indium
Indium
Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two...
, zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
allow formation of metastable δ state when rapidly cooled. High amount of hafnium
Hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable...
, holmium
Holmium
Holmium is a chemical element with the symbol Ho and atomic number 67. Part of the lanthanide series, holmium is a rare earth element. Its oxide was first isolated from rare earth ores in 1878 and the element was named after the city of Stockholm....
and thallium
Thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
also allows retaining some of the δ phase at room temperature. Neptunium
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
is the only element that can stabilize the α phase at higher temperatures. Titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, hafnium
Hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable...
and zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
stabilize the β phase at room temperature when rapidly cooled.

