Aluminium hydride
Encyclopedia
Aluminium hydride is an inorganic compound
with the formula
Al
H
3. It is a colourless solid that is pyrophoric. Although rarely encountered outside of research laboratory, alane and its derivatives are used as reducing agent
s in organic synthesis
.
s, which are named α-alane, α’-alane, β-alane, δ-alane, ε-alane, θ-alane, and γ-alane. α-Alane has a cubic or rhombohedral morphology, whereas α’-alane forms needle like crystals and γ-alane forms a bundle of fused needles. Alane is soluble in THF and ether, and its precipitation rate from ether depends on the preparation method.
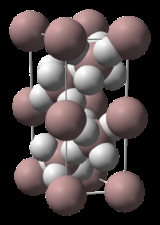
The structure of α-alane has been determined and features aluminium atoms surrounded by 6 hydrogen atoms that bridge to 6 other aluminium atoms. The Al-H distances are all equivalent (172pm) and the Al-H-Al angle is 141°.
α-Alane is the most thermally stable polymorph. β-alane and γ-alane are produced together, and convert to α-alane upon heating. δ, ε, and θ-alane are produced in different crystallization condition. Although they are less thermally stable, they do not convert into α-alane upon heating.
matrix and shown to be planar. The dimer Al2H6 has been isolated in solid hydrogen and is isostructural with diborane
,B2H6, and digallane
, Ga2H6.
s and ether
complexes have long been reported.. Its first synthesis published in 1947 and a U.S. patent for the synthesis was assigned to Petrie et al. in 1999. Aluminium hydride is prepared by treating lithium aluminium hydride
with aluminium trichloride. The procedure is intricate, attention must be given to the removal of lithium chloride
.
The ether solution of alane requires immediate use, because polymeric material precipitate otherwise. Aluminium hydride solutions are known to degrade after 3 days. Aluminium hydride is more reactive than LiAlH4, but their handling properties are similar.
Several other methods exist for the preparation of aluminium hydride:
1. AlH4- - e- → AlH3.nTHF + ½H2
For soluble anodes, anodic dissolution is expected according to reaction 2,
2. 3AlH4- + Al - 3e- → 4AlH3.nTHF
In reaction 2, the aluminum anode is consumed, limiting the production of aluminum hydride for a given electrochemical cell.
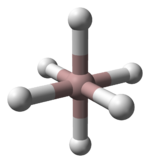
. The 1:1 complex is tetrahedral in the gas phase, but in the solid phase it is dimeric with bridging hydrogen centres, (NMe3Al(μ-H))2. The 1:2 complex adopts a trigonal bipyramidal structure
. Some adducts (e.g. dimethylethylamine alane, NMe2Et.AlH3) thermally decompose to give aluminium metal and may have use in MOCVD applications.
Its complex with diethyl ether
forms according to the following stoichiometry:
The reaction with lithium hydride
in ether produces lithium aluminium hydride
:
. Aluminium hydride will reduce aldehyde
s, ketone
s, carboxylic acid
s, anhydrides, acid chlorides, ester
s, and lactone
s to their corresponding alcohol
s. Amide
s, nitrile
s, and oxime
s are reduced to their corresponding amine
s.
In terms of functional group selectivity, alane differs from other hydride reagents. For example, in the following cyclohexanone reduction, lithium aluminium hydride
gives a trans:cis ratio of 1.9 : 1, whereas aluminium hydride gives a trans:cis ratio of 7.3 : 1.
Alane enables the hydroxymethylation of certain ketones, that is the replacement of C-H by C-CH2OH). The ketone itself is not reduced as it is "protected" as its enolate.
Organohalides are reduced slowly or not at all by aluminium hydride. Therefore, reactive functional groups such as carboxylic acid
s can be reduced in the presence of halides.
Nitro groups are not reduced by aluminium hydride. Likewise, aluminium hydride can accomplish the reduction of an ester
in the presence of nitro groups.
Aluminium hydride can be used in the reduction of acetals to half protected diols.
Aluminium hydride can also be used in epoxide ring opening reaction as shown below.
 The allylic rearrangement reaction carried out using aluminium hydride is a SN2 reaction, and it is not sterically demanding.
The allylic rearrangement reaction carried out using aluminium hydride is a SN2 reaction, and it is not sterically demanding.
Aluminum hydride even reduces carbon dioxide
to methane
under heating:
, aluminium hydride can add across double bond
s. Hydroboration is a similar reaction.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
Al
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
3. It is a colourless solid that is pyrophoric. Although rarely encountered outside of research laboratory, alane and its derivatives are used as reducing agent
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
s in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
Structure
Alane is a polymer. Its formula is sometimes represented with the formula (AlH3)n. Aluminium hydride forms numerous polymorphPolymorphism (materials science)
Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
s, which are named α-alane, α’-alane, β-alane, δ-alane, ε-alane, θ-alane, and γ-alane. α-Alane has a cubic or rhombohedral morphology, whereas α’-alane forms needle like crystals and γ-alane forms a bundle of fused needles. Alane is soluble in THF and ether, and its precipitation rate from ether depends on the preparation method.
The structure of α-alane has been determined and features aluminium atoms surrounded by 6 hydrogen atoms that bridge to 6 other aluminium atoms. The Al-H distances are all equivalent (172pm) and the Al-H-Al angle is 141°.
 |
 |
 |
α-Alane is the most thermally stable polymorph. β-alane and γ-alane are produced together, and convert to α-alane upon heating. δ, ε, and θ-alane are produced in different crystallization condition. Although they are less thermally stable, they do not convert into α-alane upon heating.
Molecular forms of alane
Monomeric AlH3 has been isolated at low temperature in a solid noble gasNoble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
matrix and shown to be planar. The dimer Al2H6 has been isolated in solid hydrogen and is isostructural with diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
,B2H6, and digallane
Digallane
Digallane is a chemical compound of gallium and hydrogen with the formula Ga2H6. It is the dimer of the monomeric compound gallane. The eventual preparation of the pure compound, reported in 1989,...
, Ga2H6.
Preparation
Aluminium hydride impurities and related amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s and ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
complexes have long been reported.. Its first synthesis published in 1947 and a U.S. patent for the synthesis was assigned to Petrie et al. in 1999. Aluminium hydride is prepared by treating lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
with aluminium trichloride. The procedure is intricate, attention must be given to the removal of lithium chloride
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
.
- 3 LiAlH4 + AlCl3 → 4 AlH3 + 3 LiCl
The ether solution of alane requires immediate use, because polymeric material precipitate otherwise. Aluminium hydride solutions are known to degrade after 3 days. Aluminium hydride is more reactive than LiAlH4, but their handling properties are similar.
Several other methods exist for the preparation of aluminium hydride:
- 2 LiAlH4 + BeCl2 → 2 AlH3 + LiBeH2Cl2
- 2 LiAlH4 + H2SO4 → 2 AlH3 + Li2SO4 + 2 H2
- 2 LiAlH4 + ZnCl2 → 2 AlH3 + 2 LiCl + ZnH2
Electrochemical synthesis
Alane can be produced by eletrochemically.. As described in the 1962 patent, the method avoids chloride impurities. Two possible mechanisms are discussed for the formation of alane in Clasen's electrochemical cell containing THF as the solvent, sodium aluminum hydride as the electrolyte, an aluminum anode, and an iron (Fe) wire submerged in mercury (Hg) as the cathode. The sodium forms an amalgam with the Hg cathode preventing side reactions and the hydrogen produced in the first reaction could be captured and reacted back with the sodium mercury amalgam to produce sodium hydride. Clasen's system results in no loss of starting material. For an insoluble anode see reaction 1.1. AlH4- - e- → AlH3.nTHF + ½H2
For soluble anodes, anodic dissolution is expected according to reaction 2,
2. 3AlH4- + Al - 3e- → 4AlH3.nTHF
In reaction 2, the aluminum anode is consumed, limiting the production of aluminum hydride for a given electrochemical cell.
Formation of adducts with Lewis bases
AlH3 readily forms adducts with strong Lewis bases. For example, both 1:1 and 1:2 complexes form with trimethylamineTrimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
. The 1:1 complex is tetrahedral in the gas phase, but in the solid phase it is dimeric with bridging hydrogen centres, (NMe3Al(μ-H))2. The 1:2 complex adopts a trigonal bipyramidal structure
Trigonal bipyramid molecular geometry
In chemistry a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular dipyramid...
. Some adducts (e.g. dimethylethylamine alane, NMe2Et.AlH3) thermally decompose to give aluminium metal and may have use in MOCVD applications.
Its complex with diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
forms according to the following stoichiometry:
- AlH3 + (C2H5)2O → H3Al•O(C2H5)2
The reaction with lithium hydride
Lithium hydride
Lithium hydride is the inorganic compound with the formula LiH. It is a colorless solid, although commercial samples are gray. Characteristic of a salt-like, or ionic, hydride, it has a high melting point and is not soluble in any solvent with which it does not react...
in ether produces lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
:
- AlH3 + LiH → LiAlH4
Reduction of functional groups
In organic chemistry, aluminium hydride is mainly used for the reduction of functional groups. In many ways, the reactivity of aluminium hydride is similar to that of lithium aluminium hydrideLithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
. Aluminium hydride will reduce aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s, anhydrides, acid chlorides, ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s, and lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s to their corresponding alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s. Amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s, nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
s, and oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s are reduced to their corresponding amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s.
In terms of functional group selectivity, alane differs from other hydride reagents. For example, in the following cyclohexanone reduction, lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
gives a trans:cis ratio of 1.9 : 1, whereas aluminium hydride gives a trans:cis ratio of 7.3 : 1.
Alane enables the hydroxymethylation of certain ketones, that is the replacement of C-H by C-CH2OH). The ketone itself is not reduced as it is "protected" as its enolate.
Organohalides are reduced slowly or not at all by aluminium hydride. Therefore, reactive functional groups such as carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s can be reduced in the presence of halides.
Nitro groups are not reduced by aluminium hydride. Likewise, aluminium hydride can accomplish the reduction of an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
in the presence of nitro groups.
Aluminium hydride can be used in the reduction of acetals to half protected diols.
Aluminium hydride can also be used in epoxide ring opening reaction as shown below.

Aluminum hydride even reduces carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
to methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
under heating:
- 4 AlH3 + 3 CO2 → 3 CH4 + 2 Al2O3
Hydroalumination
Aluminium hydride has been shown to add to propargylic alcohols. Used together with titanium tetrachlorideTitanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
, aluminium hydride can add across double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s. Hydroboration is a similar reaction.
Fuel
Aluminium hydride have been discussed for storing hydrogen in hydrogen-fueled vehicles. AlH3 contains up to 10% hydrogen by weight, corresponding to 148g/L, twice the density of liquid H2. Unfortunately, AlH3 is not a reversible carrier of hydrogen. It is a potential additive to rocket fuel and in explosive and pyrotechnic compositions.Precautions
Aluminium hydride is not spontaneously flammable, but it is highly reactive, similar to lithium aluminium hydride. Aluminum hydride decomposes in air and water. Violent reactions occur with both.External links
- Aluminium Hydride on EnvironmentalChemistry.com Chemical Database
- Hydrogen Storage from Brookhaven National Laboratory
- Aluminum Trihydride on WebElements

