.gif)
Annealing (metallurgy)
Encyclopedia
Annealing, in metallurgy
and materials science
, is a heat treatment
wherein a material is altered, causing changes in its properties such as strength
and hardness. It is a process that produces conditions by heating to above the recrystallization temperature, maintaining a suitable temperature, and then cooling. Annealing is used to induce ductility
, soften material, relieve internal stresses, refine the structure by making it homogeneous, and improve cold working properties.
In the cases of copper
, steel
, silver
, and brass
, this process is performed by substantially heating the material (generally until glowing) for a while and allowing it to cool. Unlike ferrous metals—which must be cooled slowly to anneal—copper, silver and brass can be cooled slowly in air or quickly by quenching in water. In this fashion the metal is softened and prepared for further work such as shaping, stamping, or forming.
of atoms within a solid material, so that the material progresses towards its equilibrium state. Heat is needed to increase the rate of diffusion by providing the energy needed to break bonds. The movement of atoms has the effect of redistributing and destroying the dislocations in metals and (to a lesser extent) in ceramics. This alteration in dislocations allows metals to deform more easily, so increases their ductility.
The amount of process-initiating Gibbs free energy
in a deformed metal is also reduced by the annealing process. In practice and industry, this reduction of Gibbs free energy is termed "stress relief".
The relief of internal stresses is a thermodynamically spontaneous process
; however, at room temperatures, it is a very slow process. The high temperatures at which the annealing process occurs serve to accelerate this process.
The reaction facilitating the return of the cold-worked metal to its stress-free state has many reaction pathways, mostly involving the elimination of lattice vacancy gradients within the body of the metal. The creation of lattice vacancies is governed by the Arrhenius equation
, and the migration/diffusion of lattice vacancies are governed by Fick’s laws
of diffusion.
Mechanical properties, such as hardness and ductility, change as dislocations are eliminated and the metal's crystal lattice is altered. On heating at specific temperature and cooling it is possible to bring the atom at the right lattice site and new grain growth can improve the mechanical properties.
phase, which results in softening of the metal through removal of crystal
defects (the primary type of which is the linear defect called a dislocation) and the internal stresses which they cause. Recovery phase covers all annealing phenomena that occur before the appearance of new strain-free grains. The second phase is recrystallization
, where new strain-free grains nucleate and grow to replace those deformed by internal stresses. If annealing is allowed to continue once recrystallization has been completed, grain growth
will occur, in which the microstructure starts to coarsen and may cause the metal to have less than satisfactory mechanical properties.
(a mixture of carbon monoxide, hydrogen gas, and nitrogen gas). Annealing is also done in forming gas
, a mixture of hydrogen and nitrogen.
The magnet
ic properties of mu-metal
(Espey cores) are introduced by annealing the alloy in a hydrogen
atmosphere
.
industry, silicon
wafers are annealed, so that dopant
atoms, usually boron
, phosphorus
or arsenic
, can diffuse into substitutional positions in the crystal lattice, resulting in drastic changes in the electrical properties of the semiconducting material.
It can also be referred to as: Heating a ferrous
alloy to a suitable temperature above the transformation temperature range and cooling in air to a temperature substantially below the transformation range.
This process is typically confined to hardenable steel. It is used to refine grains which have been deformed through cold work, and can improve ductility and toughness of the steel. It involves heating the steel to just above its upper critical point. It is soaked for a short period then allowed to cool in air. Small grains are formed which give a much harder and tougher metal with normal tensile strength and not the maximum ductility achieved by annealing. It eliminates columnar
grains and dendritic segregation that sometimes occurs during casting. Normalizing improves machinability
of a component and provides dimensional stability if subjected to further heat treatment processes.
, drawing
, forging
, spinning
, extruding and heading
. The piece is heated to a temperature typically below the austenizing
temperature, and held there long enough to relieve stresses in the metal. The piece is finally cooled slowly to room temperature. It is then ready again for additional cold working. This can also be used to ensure there is reduced risk of distortion of the work piece during machining, welding, or further heat treatment cycles.
The temperature range for process annealing ranges from 260 °C(500 °F) to 760 °C(1400 °F), depending on the alloy in question.
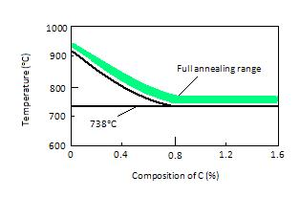 A full anneal typically results in the second most ductile state a metal can assume for metal alloy. It creates an entirely new homogeneous and uniform structure with good dynamic properties. To perform a full anneal on steel for example, steel is heated to its annealing point (about 50°C above the austenic temperature as graph shows) and held for sufficient time to allow the material to fully austenitize, to form austenite or austenite-cementite grain structure. The material is then allowed to cool slowly so that the equilibrium
A full anneal typically results in the second most ductile state a metal can assume for metal alloy. It creates an entirely new homogeneous and uniform structure with good dynamic properties. To perform a full anneal on steel for example, steel is heated to its annealing point (about 50°C above the austenic temperature as graph shows) and held for sufficient time to allow the material to fully austenitize, to form austenite or austenite-cementite grain structure. The material is then allowed to cool slowly so that the equilibrium
microstructure is obtained. In some cases this means the material is allowed to air cool. In other cases the material is allowed to furnace cool. The details of the process depend on the type of metal and the precise alloy involved. In any case the result is a more ductile material that has greater stretch ratio and reduction of area properties but a lower yield strength and a lower tensile strength
. This process is also called LP annealing for lamellar pearlite in the steel industry as opposed to a process anneal which does not specify a microstructure and only has the goal of softening the material. Often material that is to be machined, will be annealed, then be followed by further heat treatment to obtain the final desired properties.
. It can be advantageous because it does not require a temperature
-regulated furnace
like other methods of annealing.
The process consists of two conductive pulley
s (step pulleys) which the wire passes across after it is drawn. The two pulleys have an electrical potential across them, which causes the wire to form a short circuit. The Joule effect causes the temperature of the wire to rise to approximately 400 °C. This temperature is affected by the rotational speed of the pulleys, the ambient temperature, and the voltage applied. Where t is the temperature of the wire, K is a constant, V is the voltage
applied, r is the number of rotations of the pulleys per minute, and ta is the ambient temperature:
t = ((KV ²)/(r))+ta
The constant K depends on the diameter of the pulleys and the resistivity of the copper.
Purely in terms of the temperature of the copper wire, an increase in the speed with which the wire passes through the pulley system has the same effect as an increase in resistance. Therefore, the speed with which the wire can be drawn through varies quadratically as the voltage applied.
Metallurgy
Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys. It is also the technology of metals: the way in which science is applied to their practical use...
and materials science
Materials science
Materials science is an interdisciplinary field applying the properties of matter to various areas of science and engineering. This scientific field investigates the relationship between the structure of materials at atomic or molecular scales and their macroscopic properties. It incorporates...
, is a heat treatment
Heat treatment
Heat treating is a group of industrial and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass...
wherein a material is altered, causing changes in its properties such as strength
Strength of materials
In materials science, the strength of a material is its ability to withstand an applied stress without failure. The applied stress may be tensile, compressive, or shear. Strength of materials is a subject which deals with loads, deformations and the forces acting on a material. A load applied to a...
and hardness. It is a process that produces conditions by heating to above the recrystallization temperature, maintaining a suitable temperature, and then cooling. Annealing is used to induce ductility
Ductility
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
, soften material, relieve internal stresses, refine the structure by making it homogeneous, and improve cold working properties.
In the cases of copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
, silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
, and brass
Brass
Brass is an alloy of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties.In comparison, bronze is principally an alloy of copper and tin...
, this process is performed by substantially heating the material (generally until glowing) for a while and allowing it to cool. Unlike ferrous metals—which must be cooled slowly to anneal—copper, silver and brass can be cooled slowly in air or quickly by quenching in water. In this fashion the metal is softened and prepared for further work such as shaping, stamping, or forming.
Thermodynamics
Annealing occurs by the diffusionDiffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of atoms within a solid material, so that the material progresses towards its equilibrium state. Heat is needed to increase the rate of diffusion by providing the energy needed to break bonds. The movement of atoms has the effect of redistributing and destroying the dislocations in metals and (to a lesser extent) in ceramics. This alteration in dislocations allows metals to deform more easily, so increases their ductility.
The amount of process-initiating Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
in a deformed metal is also reduced by the annealing process. In practice and industry, this reduction of Gibbs free energy is termed "stress relief".
The relief of internal stresses is a thermodynamically spontaneous process
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
; however, at room temperatures, it is a very slow process. The high temperatures at which the annealing process occurs serve to accelerate this process.
The reaction facilitating the return of the cold-worked metal to its stress-free state has many reaction pathways, mostly involving the elimination of lattice vacancy gradients within the body of the metal. The creation of lattice vacancies is governed by the Arrhenius equation
Arrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
, and the migration/diffusion of lattice vacancies are governed by Fick’s laws
Fick's law of diffusion
Fick's laws of diffusion describe diffusion and can be used to solve for the diffusion coefficient, D. They were derived by Adolf Fick in the year 1855.- Fick's first law :...
of diffusion.
Mechanical properties, such as hardness and ductility, change as dislocations are eliminated and the metal's crystal lattice is altered. On heating at specific temperature and cooling it is possible to bring the atom at the right lattice site and new grain growth can improve the mechanical properties.
Stages
There are three stages in the annealing process, with the first being the recoveryRecovery (metallurgy)
Recovery is a process by which deformed grains can reduce their stored energy by the removal or rearrangement of defects in their crystal structure. These defects, primarily dislocations, are introduced by plastic deformation of the material and act to increase the yield strength of a material...
phase, which results in softening of the metal through removal of crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
defects (the primary type of which is the linear defect called a dislocation) and the internal stresses which they cause. Recovery phase covers all annealing phenomena that occur before the appearance of new strain-free grains. The second phase is recrystallization
Recrystallization (metallurgy)
Recrystallization is a process by which deformed grains are replaced by a new set of undeformed grains that nucleate and grow until the original grains have been entirely consumed. Recrystallization is usually accompanied by a reduction in the strength and hardness of a material and a simultaneous...
, where new strain-free grains nucleate and grow to replace those deformed by internal stresses. If annealing is allowed to continue once recrystallization has been completed, grain growth
Grain growth
Grain growth is the increase in size of grains in a material at high temperature. This occurs when recovery and recrystallisation are complete and further reduction in the internal energy can only be achieved by reducing the total area of grain boundary...
will occur, in which the microstructure starts to coarsen and may cause the metal to have less than satisfactory mechanical properties.
Controlled atmospheres
The high temperature of annealing may result in oxidation of the metal’s surface, resulting in scale. If scale is to be avoided, annealing is carried out in a special atmosphere, such as with endothermic gasEndothermic gas
Endothermic gas is the gaseous product of incomplete combustion in a controlled environment with a composition of hydrogen gas , nitrogen gas , and carbon monoxide . Hydrogen and carbon monoxide are reducing agents, so they shield surfaces from oxidation....
(a mixture of carbon monoxide, hydrogen gas, and nitrogen gas). Annealing is also done in forming gas
Forming gas
Forming gas is a mixture of hydrogen and nitrogen. It is sometimes called a "dissociated ammonia atmosphere" due to the reaction which generates it:...
, a mixture of hydrogen and nitrogen.
The magnet
Magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, and attracts or repels other magnets.A permanent magnet is an object...
ic properties of mu-metal
Mu-metal
Mu-metal is a nickel-iron alloy that is notable for its high magnetic permeability. The high permeability makes mu-metal very effective at screening static or low-frequency magnetic fields, which cannot be attenuated by other methods. The name came from the Greek letter mu which represents...
(Espey cores) are introduced by annealing the alloy in a hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atmosphere
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
.
Setup and equipment
Typically, large ovens are used for the annealing process. The inside of the oven is large enough to place the workpiece in a position to receive maximum exposure to the circulating heated air. For high volume process annealing, gas fired conveyor furnaces are often used. For large workpieces or high quantity parts Car-bottom furnaces will be used in order to move the parts in and out with ease. Once the annealing process has been successfully completed, the workpieces are sometimes left in the oven in order for the parts to have a controlled cooling process. While some workpieces are left in the oven to cool in a controlled fashion, other materials and alloys are removed from the oven. After being removed from the oven, the workpieces are often quickly cooled off in a process known as quench hardening. Some typical methods of quench hardening materials involve the use of media such as air, water, oil, or salt. Quench hardening is generally applicable to some ferrous alloys, but not copper alloys.Diffusion annealing of semiconductors
In the semiconductorSemiconductor
A semiconductor is a material with electrical conductivity due to electron flow intermediate in magnitude between that of a conductor and an insulator. This means a conductivity roughly in the range of 103 to 10−8 siemens per centimeter...
industry, silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
wafers are annealed, so that dopant
Dopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
atoms, usually boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
, phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
or arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
, can diffuse into substitutional positions in the crystal lattice, resulting in drastic changes in the electrical properties of the semiconducting material.
Normalization
Normalization is an annealing process in which a metal is cooled in air after heating in order to relieve stress.It can also be referred to as: Heating a ferrous
Ferrous
Ferrous , in chemistry, indicates a divalent iron compound , as opposed to ferric, which indicates a trivalent iron compound ....
alloy to a suitable temperature above the transformation temperature range and cooling in air to a temperature substantially below the transformation range.
This process is typically confined to hardenable steel. It is used to refine grains which have been deformed through cold work, and can improve ductility and toughness of the steel. It involves heating the steel to just above its upper critical point. It is soaked for a short period then allowed to cool in air. Small grains are formed which give a much harder and tougher metal with normal tensile strength and not the maximum ductility achieved by annealing. It eliminates columnar
Columnar
In biology, columnar refers to the shape of epithelial cells that are taller than they are wide. Form follows function in biology, and columnar morphorphology hints at the functions of the cell. Columnar cells are important in absorption and movement of mucus...
grains and dendritic segregation that sometimes occurs during casting. Normalizing improves machinability
Machinability
The term machinability refers to the ease with which a metal can be machined to an acceptable surface finish. Materials with good machinability require little power to cut, can be cut quickly, easily obtain a good finish, and do not wear the tooling much; such materials are said to be free machining...
of a component and provides dimensional stability if subjected to further heat treatment processes.
Process annealing
Process annealing, also called "intermediate annealing", "subcritical annealing", or "in-process annealing", is a heat treatment cycle that restores some of the ductility to a work piece allowing it be worked further without breaking. Ductility is important in shaping and creating a more refined piece of work through processes such as rollingRolling (metalworking)
In metalworking, rolling is a metal forming process in which metal stock is passed through a pair of rolls. Rolling is classified according to the temperature of the metal rolled. If the temperature of the metal is above its recrystallization temperature, then the process is termed as hot rolling...
, drawing
Drawing (manufacturing)
Drawing is a metalworking process which uses tensile forces to stretch metal. It is broken up into two types: sheet metal drawing and wire, bar, and tube drawing. The specific definition for sheet metal drawing is that it involves plastic deformation over a curved axis...
, forging
Forging
Forging is a manufacturing process involving the shaping of metal using localized compressive forces. Forging is often classified according to the temperature at which it is performed: '"cold," "warm," or "hot" forging. Forged parts can range in weight from less than a kilogram to 580 metric tons...
, spinning
Metal spinning
Metal spinning, also known as spin forming or spinning, is a metalworking process by which a disc or tube of metal is rotated at high speed and formed into an axially symmetric part. Spinning can be performed by hand or by a CNC lathe....
, extruding and heading
Heading (metalworking)
Heading is a metalworking process which incorporates the forging, extruding and upsetting process. It is often performed in the cold state, resulting in cold working...
. The piece is heated to a temperature typically below the austenizing
Austenite
Austenite, also known as gamma phase iron, is a metallic non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of ; other alloys of steel have different eutectoid temperatures...
temperature, and held there long enough to relieve stresses in the metal. The piece is finally cooled slowly to room temperature. It is then ready again for additional cold working. This can also be used to ensure there is reduced risk of distortion of the work piece during machining, welding, or further heat treatment cycles.
The temperature range for process annealing ranges from 260 °C(500 °F) to 760 °C(1400 °F), depending on the alloy in question.
Full anneal

Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
microstructure is obtained. In some cases this means the material is allowed to air cool. In other cases the material is allowed to furnace cool. The details of the process depend on the type of metal and the precise alloy involved. In any case the result is a more ductile material that has greater stretch ratio and reduction of area properties but a lower yield strength and a lower tensile strength
Tensile strength
Ultimate tensile strength , often shortened to tensile strength or ultimate strength, is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract...
. This process is also called LP annealing for lamellar pearlite in the steel industry as opposed to a process anneal which does not specify a microstructure and only has the goal of softening the material. Often material that is to be machined, will be annealed, then be followed by further heat treatment to obtain the final desired properties.
Short cycle anneal
Short cycle annealing is used for turning normal ferrite into malleable ferrite. It consists of heating, cooling, and then heating again from 4 to 8 hours.Resistive heating
Resistive heating can be used to efficiently anneal copper wire; the heating system employs a controlled electrical short circuitShort circuit
A short circuit in an electrical circuit that allows a current to travel along an unintended path, often where essentially no electrical impedance is encountered....
. It can be advantageous because it does not require a temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
-regulated furnace
Furnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
like other methods of annealing.
The process consists of two conductive pulley
Pulley
A pulley, also called a sheave or a drum, is a mechanism composed of a wheel on an axle or shaft that may have a groove between two flanges around its circumference. A rope, cable, belt, or chain usually runs over the wheel and inside the groove, if present...
s (step pulleys) which the wire passes across after it is drawn. The two pulleys have an electrical potential across them, which causes the wire to form a short circuit. The Joule effect causes the temperature of the wire to rise to approximately 400 °C. This temperature is affected by the rotational speed of the pulleys, the ambient temperature, and the voltage applied. Where t is the temperature of the wire, K is a constant, V is the voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
applied, r is the number of rotations of the pulleys per minute, and ta is the ambient temperature:
t = ((KV ²)/(r))+ta
The constant K depends on the diameter of the pulleys and the resistivity of the copper.
Purely in terms of the temperature of the copper wire, an increase in the speed with which the wire passes through the pulley system has the same effect as an increase in resistance. Therefore, the speed with which the wire can be drawn through varies quadratically as the voltage applied.
See also
- Annealing (glass)Annealing (glass)Annealing is a process of slowly cooling glass to relieve internal stresses after it was formed. The process may be carried out in a temperature-controlled kiln known as a Lehr. Glass which has not been annealed is liable to crack or shatter when subjected to a relatively small temperature change...
- Hollomon-Jaffe parameterHollomon-Jaffe parameterThe Hollomon-Jaffe parameter, or HP, describes the effect of a heat treatment at a temperature for a certain time.-Effect:The effect of the heat treatment depends on its temperature and its time...
- Low hydrogen annealingLow hydrogen annealingLow hydrogen annealing is a heat treatment in metallurgy for the reduction or elimination of hydrogen in a material to prevent hydrogen embrittlement.-Process description:...
- TemperingTemperingTempering is a heat treatment technique for metals, alloys and glass. In steels, tempering is done to "toughen" the metal by transforming brittle martensite or bainite into a combination of ferrite and cementite or sometimes Tempered martensite...
Further reading
- Thesis of Degree, Cable Manufacture and Tests of General Use and Energy. - Jorge Luis Pedraz (1994), UNI, Files, Peru.
- Dynamic annealing of the Copper wire by using a Controlled Short circuit. = Jorge Luis Pedraz (1999), Peru: Lima , CONIMERA 1999, INTERCON 99,
External links
- Annealing with induction: Ameritherm offers annealing overview and Application Notes
- Annealing:efunda - engineering fundamentals
- Full Annealing:Material Science
- Annealing: Aluminum and Aircraft Metal Alloys

