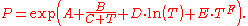Antoine equation
Encyclopedia
The Antoine equation
is a vapor pressure equation and describes the relation between vapor pressure
and temperature for pure components. The Antoine equation is derived from the Clausius-Clapeyron relation
.
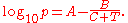
where p is the vapor pressure, T is temperature
and A, B and C are component-specific constants.
The simplified form with C set to zero: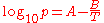
is named the August equation, after the German physicist Ernst Ferdinand August (1795–1870). The August equation describes a linear relation between the logarithm of the pressure and the reciprocal temperature. This assumes a temperature-independent heat of vaporization. The Antoine equation allows an improved, but still inexact description of the change of the heat of vaporization with the temperature.
The Antoine equation can also be transformed in a temperature-explicit form with simple algebraic manipulations: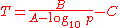
to the critical point
, because it is not flexible enough. Therefore, multiple parameter sets for a single component are commonly used. A low-pressure parameter set is used to describe the vapour pressure curve up to the normal boiling point and the second set of parameters is used for the range from the normal boiling point to the critical point.
The constants are given in °C and mmHg.
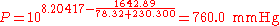
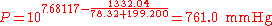
(760 mmHg = 101.325 kPa = 1.000 atm = normal pressure)
This example shows a severe problem caused by using two different sets of coefficients. The described vapor pressure is not continuous
—at the normal boiling point the two sets give different results. This causes severe problems for computational techniques which rely on a continuous vapor pressure curve.
Two solutions are possible: The first approach uses a single Antoine parameter set over a larger temperature range and accepts the increased deviation between calculated and real vapor pressures. A variant of this single set approach is using a special parameter set fitted for the examined temperature range. The second solution is switching to another vapor pressure equation with more than three parameters. Commonly used are simple extensions of the Antoine equation (see below) and the equations of DIPPR or Wagner.
is recommended and pascal
s are preferred. The usage of the pre-SI units has only historic reasons and originates directly from Antoine's original publication.
It is however easy to convert the parameters to different pressure and temperature units. For switching from degrees Celsius to kelvin it is sufficient to subtract 273.15 from the C parameter. For switching from millimeters of mercury to pascals it is sufficient to add the common logarithm
of the factor between both units to the A parameter:
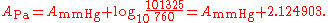
The parameters for °C and mmHg for ethanol
are converted for K and Pa to
The first example calculation with TB = 351.47 K becomes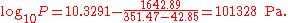
A similarly simple transformation can be used if the common logarithm should be exchanged by the natural logarithm. It is sufficient to multiply the A and B parameters by ln(10) = 2.302585.
The example calculation with the converted parameters (for K and Pa):
becomes
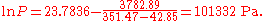
(The small differences in the results are only caused by the used limited precision of the coefficients).
The additional parameters increase the flexibility of the equation and allow the description of the entire vapor pressure curve. The extended equation forms can be reduced to the original form by setting the additional parameters D, E and F to 0.
A further difference is that the extended equations use the e as base for the exponential function and the natural logarithm. This doesn't affect the equation form.
See also the Wagner Equation
is a vapor pressure equation and describes the relation between vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and temperature for pure components. The Antoine equation is derived from the Clausius-Clapeyron relation
Clausius-Clapeyron relation
The Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît Paul Émile Clapeyron, who defined it sometime after 1834, is a way of characterizing a discontinuous phase transition between two phases of matter. On a pressure–temperature diagram, the line separating the two phases is known...
.
The equation
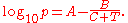
where p is the vapor pressure, T is temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and A, B and C are component-specific constants.
The simplified form with C set to zero:
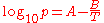
is named the August equation, after the German physicist Ernst Ferdinand August (1795–1870). The August equation describes a linear relation between the logarithm of the pressure and the reciprocal temperature. This assumes a temperature-independent heat of vaporization. The Antoine equation allows an improved, but still inexact description of the change of the heat of vaporization with the temperature.
The Antoine equation can also be transformed in a temperature-explicit form with simple algebraic manipulations:
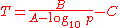
Validity range
Usually, the Antoine equation cannot be used to describe the entire saturated vapour pressure curve from the triple pointTriple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
to the critical point
Critical point (thermodynamics)
In physical chemistry, thermodynamics, chemistry and condensed matter physics, a critical point, also called a critical state, specifies the conditions at which a phase boundary ceases to exist...
, because it is not flexible enough. Therefore, multiple parameter sets for a single component are commonly used. A low-pressure parameter set is used to describe the vapour pressure curve up to the normal boiling point and the second set of parameters is used for the range from the normal boiling point to the critical point.
Example parameters
| A | B | C | T min. °C |
T max °C |
|
|---|---|---|---|---|---|
| Water | 8.07131 | 1730.63 | 233.426 | 1 | 100 |
| Water | 8.14019 | 1810.94 | 244.485 | 99 | 374 |
| Ethanol | 8.20417 | 1642.89 | 230.300 | ||
| 80 | |||||
| Ethanol | 7.68117 | 1332.04 | 199.200 | 77 | 243 |
The constants are given in °C and mmHg.
Example calculation
The normal boiling point of ethanol is TB = 78.32 °C.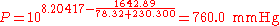
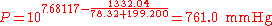
(760 mmHg = 101.325 kPa = 1.000 atm = normal pressure)
This example shows a severe problem caused by using two different sets of coefficients. The described vapor pressure is not continuous
Continuous function
In mathematics, a continuous function is a function for which, intuitively, "small" changes in the input result in "small" changes in the output. Otherwise, a function is said to be "discontinuous". A continuous function with a continuous inverse function is called "bicontinuous".Continuity of...
—at the normal boiling point the two sets give different results. This causes severe problems for computational techniques which rely on a continuous vapor pressure curve.
Two solutions are possible: The first approach uses a single Antoine parameter set over a larger temperature range and accepts the increased deviation between calculated and real vapor pressures. A variant of this single set approach is using a special parameter set fitted for the examined temperature range. The second solution is switching to another vapor pressure equation with more than three parameters. Commonly used are simple extensions of the Antoine equation (see below) and the equations of DIPPR or Wagner.
Units
The coefficients of Antoine's equation are normally given in mmHg—even today where the SIInternational System of Units
The International System of Units is the modern form of the metric system and is generally a system of units of measurement devised around seven base units and the convenience of the number ten. The older metric system included several groups of units...
is recommended and pascal
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
s are preferred. The usage of the pre-SI units has only historic reasons and originates directly from Antoine's original publication.
It is however easy to convert the parameters to different pressure and temperature units. For switching from degrees Celsius to kelvin it is sufficient to subtract 273.15 from the C parameter. For switching from millimeters of mercury to pascals it is sufficient to add the common logarithm
Logarithm
The logarithm of a number is the exponent by which another fixed value, the base, has to be raised to produce that number. For example, the logarithm of 1000 to base 10 is 3, because 1000 is 10 to the power 3: More generally, if x = by, then y is the logarithm of x to base b, and is written...
of the factor between both units to the A parameter:
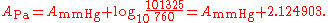
The parameters for °C and mmHg for ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
| A | B | C |
|---|---|---|
| 8.20417 | 1642.89 | 230.300 |
are converted for K and Pa to
| A | B | C |
|---|---|---|
| 10.32907 | 1642.89 | |
The first example calculation with TB = 351.47 K becomes
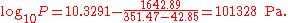
A similarly simple transformation can be used if the common logarithm should be exchanged by the natural logarithm. It is sufficient to multiply the A and B parameters by ln(10) = 2.302585.
The example calculation with the converted parameters (for K and Pa):
| A | B | C |
|---|---|---|
| 23.7836 | 3782.89 | |
becomes
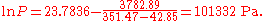
(The small differences in the results are only caused by the used limited precision of the coefficients).
Extension of the Antoine equations
To overcome the limits of the Antoine equation some simple extension by additional terms are used:The additional parameters increase the flexibility of the equation and allow the description of the entire vapor pressure curve. The extended equation forms can be reduced to the original form by setting the additional parameters D, E and F to 0.
A further difference is that the extended equations use the e as base for the exponential function and the natural logarithm. This doesn't affect the equation form.
See also the Wagner Equation
Sources for Antoine equation parameters
- NIST Chemistry WebBook
- Dortmund Data BankDortmund Data BankThe Dortmund Data Bank is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimization of chemical processes...
- Directory of reference books and data banks containing Antoine constants
- Several reference books and publications, e. g.
- Lange's Handbook of Chemistry, McGraw-Hill Professional
- Wichterle I., Linek J., "Antoine Vapor Pressure Constants of Pure Compounds"
- Yaws C. L., Yang H.-C., "To Estimate Vapor Pressure Easily. Antoine Coefficients Relate Vapor Pressure to Temperature for Almost 700 Major Organic Compounds", Hydrocarbon Processing, 68(10), Pages 65–68, 1989