Arrestin
Encyclopedia
Arrestins are a small family of protein
s important for regulating signal transduction
.
s (GPCRs) in the visual rhodopsin system by Hermann Kühn and co-workers and in the β-adrenergic system by Martin J. Lohse
and co-workers. In response to a stimulus, GPCRs activate heterotrimeric G protein
s. In order to turn off this response, or adapt to a persistent stimulus, activated receptors need to be silenced. The first step is phosphorylation
by a class of serine/threonine kinases called G protein coupled receptor kinases
(GRKs). GRK phosphorylation specifically prepares the activated receptor for arrestin binding. Arrestin binding to the receptor blocks further G protein-mediated signaling, targets receptors for internalization, and redirects signaling to alternative G protein-independent pathways. In addition to GPCRs, arrestins bind to other classes of cell surface receptors and a variety of other signaling proteins.
Fish and other vertebrates appear to have only three arrestins: no equivalent of arrestin-2, which is the most abundant non-visual subtype in mammals, was cloned so far. The proto-chordate
C. intestinalis
(sea squirt) has only one arrestin, which serves as visual in its mobile larva with highly developed eyes, and becomes generic non-visual in the blind sessile adult. Conserved positions of multiple introns in its gene and those of our arrestin subtypes suggest that they all evolved from this ancestral arrestin. Lower invertebrates, such as roundworm C. elegans
, also have only one arrestin. Insects have arr1 and arr2, originally termed “visual arrestins” because they are expressed in photoreceptors, and one non-visual subtype (kurtz in Drosophila
). Later arr1 and arr2 were found to play an important role in olfactory neurons and renamed “sensory”. Fungi have distant arrestin relatives involved in pH sensing.
and clathrin adaptor AP2
, which promotes receptor internalization via coated pits and subsequent transport to an internal compartments, called endosomes. Subsequently, the receptor could be either directed to degradation compartments (lysosome
s) or recycled back to the plasma membrane where it can once more act as a signal. The strength of arrestin-receptor interaction plays a role in this choice: tighter complexes tend to increase the probability of receptor degradation, whereas more transient complexes favor recycling, although this “rule” is far from absolute.
s (part of the cellular “skeleton”), where they assume yet another conformation, different from both free and receptor-bound form. Microtubule-bound arrestins recruit certain proteins to the cytoskeleton, which affects their activity and/or redirects it to microtubule-associated proteins.
Arrestins shuttle between cell nucleus
and cytoplasm
. Their nuclear functions are not fully understood, but it was shown that all four mammalian arrestin subtypes remove some of their partners, such as protein kinase JNK3
or the ubiquitin ligase
Mdm2
, from the nucleus. Arrestins also modify gene expression by enhancing transcription of certain genes.
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s important for regulating signal transduction
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
.
Function
Arrestins were first discovered as a part of a conserved two-step mechanism for regulating the activity of G protein-coupled receptorG protein-coupled receptor
G protein-coupled receptors , also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein-linked receptors , comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal...
s (GPCRs) in the visual rhodopsin system by Hermann Kühn and co-workers and in the β-adrenergic system by Martin J. Lohse
Martin J. Lohse
Martin J. Lohse, M.D. born August 26th, 1956, is a German physician and pharmacologist doing research on G protein-coupled receptors. Since 1993, he is a Professor of Pharmacology at the University of Würzburg, Germany, as well as Chairman of the Rudolf Virchow Center . He received his exam in...
and co-workers. In response to a stimulus, GPCRs activate heterotrimeric G protein
G protein
G proteins are a family of proteins involved in transmitting chemical signals outside the cell, and causing changes inside the cell. They communicate signals from many hormones, neurotransmitters, and other signaling factors. G protein-coupled receptors are transmembrane receptors...
s. In order to turn off this response, or adapt to a persistent stimulus, activated receptors need to be silenced. The first step is phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
by a class of serine/threonine kinases called G protein coupled receptor kinases
G-protein coupled receptor kinases
G protein-coupled receptor kinases are a family of protein kinases which regulate the activity of G protein-coupled receptors by phosphorylating their intracellular domains after their associated G proteins have been released and activated...
(GRKs). GRK phosphorylation specifically prepares the activated receptor for arrestin binding. Arrestin binding to the receptor blocks further G protein-mediated signaling, targets receptors for internalization, and redirects signaling to alternative G protein-independent pathways. In addition to GPCRs, arrestins bind to other classes of cell surface receptors and a variety of other signaling proteins.
Subtypes
Mammals express four arrestin subtypes and each arrestin subtype is known by multiple aliases. The systematic arrestin name (1-4) plus the most widely used aliases for each arrestin subtype are listed in bold below:- Arrestin-1SAG (gene)S-arrestin is a protein that in humans is encoded by the SAG gene.-Further reading:...
was originally identified as the S-antigen (SAG) causing uveitis (autoimmune eye disease), then independently described as a 48 kDa protein that binds light-activated phosphorylated rhodopsin before it became clear that both are one and the same. It was later renamed visual arrestin, but when another cone-specific visual subtype was cloned the term rod arrestin was coined. This also turned out to be a misnomer: arrestin-1 expresses at comparable very high levels in both rod and cone photoreceptor cells.
- Arrestin-2Arrestin beta 1Arrestin, beta 1, also known as ARRB1, is a protein which in humans is encoded by the ARRB1 gene.- Function :Members of arrestin/beta-arrestin protein family are thought to participate in agonist-mediated desensitization of G protein-coupled receptors and cause specific dampening of cellular...
was the first non-visual arrestin cloned. It was first named β-arrestin simply because between two GPCRsG protein-coupled receptorG protein-coupled receptors , also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein-linked receptors , comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal...
available in purified form at the time, rhodopsinRhodopsinRhodopsin, also known as visual purple, is a biological pigment of the retina that is responsible for both the formation of the photoreceptor cells and the first events in the perception of light. Rhodopsins belong to the G-protein coupled receptor family and are extremely sensitive to light,...
and β2-adrenergic receptorBeta-2 adrenergic receptorThe beta-2 adrenergic receptor , also known as ADRB2, is a beta-adrenergic receptor, and also denotes the human gene encoding it.-Gene:The gene is intronless...
, it showed preference for the latter.
- Arrestin-3Arrestin beta 2Beta-arrestin-2, also known as arrestin beta-2, is an intracellular protein that in humans is encoded by the ARRB2 gene.Members of arrestin/beta-arrestin protein family are thought to participate in agonist-mediated desensitization of G protein-coupled receptors and cause specific dampening of...
. The second non-visual arrestin cloned was first termed β-arrestin-2 (retroactively changing the name of β-arrestin into β-arrestin-1), even though by that time it was clear that non-visual arrestins interact with hundreds of different GPCRs, not just with β2-adrenergic receptor. Systematic names, arrestin-2 and arrestin-3, respectively, were proposed soon after that.
- Arrestin-4ARR3Arrestin-C also known as retinal cone arrestin-3 is a protein that in humans is encoded by the ARR3 gene.-Further reading:...
was cloned by two groups and termed cone arrestin, after photoreceptor type that expresses it, and X-arrestin, after the chromosome where its gene resides. In the HUGOHUGO Gene Nomenclature CommitteeThe HUGO Gene Nomenclature Committee approves a unique and meaningful name for every known human gene based on a query of experts. In addition to a long name, the HGNC also assigns an abbreviation to every gene...
database its gene is called arrestin-3.
Fish and other vertebrates appear to have only three arrestins: no equivalent of arrestin-2, which is the most abundant non-visual subtype in mammals, was cloned so far. The proto-chordate
Chordate
Chordates are animals which are either vertebrates or one of several closely related invertebrates. They are united by having, for at least some period of their life cycle, a notochord, a hollow dorsal nerve cord, pharyngeal slits, an endostyle, and a post-anal tail...
C. intestinalis
Ciona intestinalis
Ciona intestinalis is a urochordata , a tunicate widely distributed in Northern European waters. As an invasive species, it has also spread to other parts of the world....
(sea squirt) has only one arrestin, which serves as visual in its mobile larva with highly developed eyes, and becomes generic non-visual in the blind sessile adult. Conserved positions of multiple introns in its gene and those of our arrestin subtypes suggest that they all evolved from this ancestral arrestin. Lower invertebrates, such as roundworm C. elegans
Caenorhabditis elegans
Caenorhabditis elegans is a free-living, transparent nematode , about 1 mm in length, which lives in temperate soil environments. Research into the molecular and developmental biology of C. elegans was begun in 1974 by Sydney Brenner and it has since been used extensively as a model...
, also have only one arrestin. Insects have arr1 and arr2, originally termed “visual arrestins” because they are expressed in photoreceptors, and one non-visual subtype (kurtz in Drosophila
Drosophila
Drosophila is a genus of small flies, belonging to the family Drosophilidae, whose members are often called "fruit flies" or more appropriately pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit...
). Later arr1 and arr2 were found to play an important role in olfactory neurons and renamed “sensory”. Fungi have distant arrestin relatives involved in pH sensing.
Tissue distribution
One or more arrestin is expressed virtually in every eukaryotic cell. In mammals, arrestin-1 and arrestin-4 are largely confined to photoreceptors, whereas arrestin-2 and arrestin-3 are ubiquitous. Neurons have the highest expression level of both non-visual subtypes. In neuronal precursors both are expressed at comparable levels, whereas in mature neurons arrestin-2 is present at 10-20 fold higher levels than arrestin-3.Mechanism
Arrestins block GPCR coupling to G proteins via two mechanisms. First, arrestin binding to the cytoplasmic tip of the receptor occludes the binding site for the heterotrimeric G-protein, preventing its activation (desensitization). Second, arrestins link the receptor to elements of the internalization machinery, clathrinClathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1975. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice...
and clathrin adaptor AP2
AP2M1
AP-2 complex subunit mu is a protein that in humans is encoded by the AP2M1 gene.-Interactions:AP2M1 has been shown to interact with CTLA-4 and Alpha-1B adrenergic receptor.-Further reading:...
, which promotes receptor internalization via coated pits and subsequent transport to an internal compartments, called endosomes. Subsequently, the receptor could be either directed to degradation compartments (lysosome
Lysosome
thumb|350px|Schematic of typical animal cell, showing subcellular components. [[Organelle]]s: [[nucleoli]] [[cell nucleus|nucleus]] [[ribosomes]] [[vesicle |vesicle]] rough [[endoplasmic reticulum]]...
s) or recycled back to the plasma membrane where it can once more act as a signal. The strength of arrestin-receptor interaction plays a role in this choice: tighter complexes tend to increase the probability of receptor degradation, whereas more transient complexes favor recycling, although this “rule” is far from absolute.
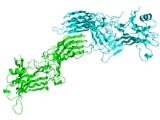
Structure
Arrestins are elongated molecules, in which several intra-molecular interactions hold the relative orientation of the two domains. In unstimulated cell arrestins are localized in the cytoplasm in this basal “inactive” conformation. Active phosphorylated GPCRs recruit arrestin to the plasma membrane. Receptor binding induces a global conformational change that involves the movement of the two arrestin domains and the release of its C-terminal tail that contains clathrin and AP2 binding sites. Increased accessibility of these sites in receptor-bound arrestin targets the arrestin-receptor complex to the coated pit. Arrestins also bind microtubuleMicrotubule
Microtubules are a component of the cytoskeleton. These rope-like polymers of tubulin can grow as long as 25 micrometers and are highly dynamic. The outer diameter of microtubule is about 25 nm. Microtubules are important for maintaining cell structure, providing platforms for intracellular...
s (part of the cellular “skeleton”), where they assume yet another conformation, different from both free and receptor-bound form. Microtubule-bound arrestins recruit certain proteins to the cytoskeleton, which affects their activity and/or redirects it to microtubule-associated proteins.
Arrestins shuttle between cell nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
and cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
. Their nuclear functions are not fully understood, but it was shown that all four mammalian arrestin subtypes remove some of their partners, such as protein kinase JNK3
MAPK10
Mitogen-activated protein kinase 10 is an enzyme that in humans is encoded by the MAPK10 gene.-Interactions:MAPK10 has been shown to interact with MAPK8IP3.-Further reading:...
or the ubiquitin ligase
Ubiquitin ligase
A ubiquitin ligase is a protein that in combination with an E2 ubiquitin-conjugating enzyme causes the attachment of ubiquitin to a lysine on a target protein via an isopeptide bond; the E3 ubiquitin ligase targets specific protein substrates for degradation by the proteasome...
Mdm2
Mdm2
Mdm2 is an important negative regulator of the p53 tumor suppressor. It is the name of a gene as well as the protein encoded by that gene. Mdm2 protein functions both as an E3 ubiquitin ligase that recognizes the N-terminal trans-activation domain of the p53 tumor suppressor and an inhibitor of...
, from the nucleus. Arrestins also modify gene expression by enhancing transcription of certain genes.

