Arsenate
Encyclopedia
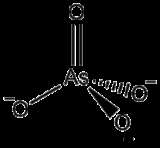
The arsenate ion
is As
O
43−.
An arsenate (compound) is any compound
that contains this ion. Arsenates are salt
s or ester
s of arsenic acid
.
The arsenic atom in arsenate has a valency of 5 and is also known as pentavalent arsenic or As[V].
Arsenate resembles phosphate
in many respects, since arsenic
and phosphorus
occur in the same group (column) of the periodic table
.
Arsenates are moderate oxidizers, with an electrode potential
of +0.56 for reduction to arsenite
s.
s. Those minerals may contain hydrated or anhydrous
arsenates. Unlike phosphates, arsenates are not lost from a mineral during weathering
. Examples of arsenate-containing minerals include adamite
, alarsite
, annabergite
, erythrite and legrandite
.
in the step of glycolysis
that produces 1,3-bisphosphoglycerate
from glyceraldehyde 3-phosphate
. This yields 1-arseno-3-phosphoglycerate instead, which is unstable and quickly hydrolyzes, forming the next intermediate in the pathway, 3-phosphoglycerate. Therefore glycolysis proceeds, but the ATP
molecule that would be generated from 1,3-bisphosphoglycerate
is lost - arsenate is an uncoupler of glycolysis, explaining its toxicity.
As with other arsenic compounds, arsenate can also inhibit the conversion of pyruvate into acetyl-CoA
, blocking the Krebs cycle and therefore resulting in further loss of ATP.
obtain their energy by oxidizing
various fuels while reducing
arsenates to form arsenite
s. The enzyme
s involved are known as arsenate reductases.
In 2008, bacteria were discovered that employ a version of photosynthesis
with arsenites as electron donor
s, producing arsenates (just like ordinary photosynthesis uses water as electron donor, producing molecular oxygen). The researchers conjectured that historically these photosynthesizing organisms produced the arsenates that allowed the arsenate-reducing bacteria to thrive.
In 2010, a team at NASA
's Astrobiology Institute
cultured samples of arsenic-resistant GFAJ-1
bacteria from Mono Lake
, using a medium high in arsenate and low in phosphate concentration. The findings suggest that the bacteria may partially incorporate arsenate in place of phosphate in some biomolecules, including DNA, but the validity of this claim is still being debated.
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
is As
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
43−.
An arsenate (compound) is any compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
that contains this ion. Arsenates are salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
s or ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s of arsenic acid
Arsenic acid
Arsenic acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but only found in solution where it...
.
The arsenic atom in arsenate has a valency of 5 and is also known as pentavalent arsenic or As[V].
Arsenate resembles phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
in many respects, since arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
and phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
occur in the same group (column) of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
.
Arsenates are moderate oxidizers, with an electrode potential
Electrode potential
Electrode potential, E, in electrochemistry, according to an IUPAC definition, is the electromotive force of a cell built of two electrodes:* on the left-hand side is the standard hydrogen electrode, and...
of +0.56 for reduction to arsenite
Arsenite
In chemistry an arsenite is a chemical compound containing an arsenic oxoanion where arsenic has oxidation state +3.The different forms of the anion are the next ones:* ortho-arsenite: AsO33-* meta-arsenite: AsO2-...
s.
Occurrence
Arsenates occur naturally in a variety of mineralMineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
s. Those minerals may contain hydrated or anhydrous
Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
arsenates. Unlike phosphates, arsenates are not lost from a mineral during weathering
Weathering
Weathering is the breaking down of rocks, soils and minerals as well as artificial materials through contact with the Earth's atmosphere, biota and waters...
. Examples of arsenate-containing minerals include adamite
Adamite
Adamite is a zinc arsenate hydroxide mineral, Zn2AsO4OH. It is a mineral that typically occurs in the oxidized or weathered zone above zinc ore occurrences. Pure adamite is colorless, but usually it possess yellow color due to Fe compounds admixture. Tints of green also occur and are connected with...
, alarsite
Alarsite
Alarsite is a mineral found in the far Eastern area of Russia. Its name is derived from its composition of Aluminium and Arsenic. Alarsite's Mohs hardness is 5-5½ and has a Trigonal crystal system.- External links :* from mindat.org* from...
, annabergite
Annabergite
Annabergite is an arsenate mineral consisting of a hydrous nickelarsenate, Ni32·8H2O, crystallizing in the monoclinic system and isomorphous with vivianite and erythrite. Crystals are minute and capillary and rarely met with, the mineral occurring usually as soft earthy masses and encrustations. A...
, erythrite and legrandite
Legrandite
Legrandite is a rare zinc arsenate mineral, Zn2·.It is an uncommon secondary mineral in the oxidized zone of zincn–arsenic bearing deposits and occurs rarely in granite pegmatite. Associated minerals include: adamite, paradamite, kottigite, scorodite, smithsonite, leiteite, renierite,...
.
Ions
- In strongly acidAcidAn acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic conditions it exists as arsenic acidArsenic acidArsenic acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but only found in solution where it...
, H3AsO4; - in weakly acidic conditions it exists as dihydrogen arsenate ion, H2AsO4−;
- in weakly basic conditions it exists as hydrogen arsenate ion HAsO42−;
- and finally, in strongly basic conditions, it exists as the arsenate ion AsO43−.
Arsenate poisoning
Arsenate can replace inorganic phosphatePhosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
in the step of glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
that produces 1,3-bisphosphoglycerate
1,3-Bisphosphoglycerate
1,3-Bisphosphoglyceric acid is a 3-carbon organic molecule present in most, if not all, living organisms. It primarily exists as a metabolic intermediate in both glycolysis during respiration and the Calvin cycle during photosynthesis...
from glyceraldehyde 3-phosphate
Glyceraldehyde 3-phosphate
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GADP, GAP, TP, GALP or PGAL, is a chemical compound that occurs as an intermediate in several central metabolic pathways of all organisms...
. This yields 1-arseno-3-phosphoglycerate instead, which is unstable and quickly hydrolyzes, forming the next intermediate in the pathway, 3-phosphoglycerate. Therefore glycolysis proceeds, but the ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
molecule that would be generated from 1,3-bisphosphoglycerate
1,3-Bisphosphoglycerate
1,3-Bisphosphoglyceric acid is a 3-carbon organic molecule present in most, if not all, living organisms. It primarily exists as a metabolic intermediate in both glycolysis during respiration and the Calvin cycle during photosynthesis...
is lost - arsenate is an uncoupler of glycolysis, explaining its toxicity.
As with other arsenic compounds, arsenate can also inhibit the conversion of pyruvate into acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
, blocking the Krebs cycle and therefore resulting in further loss of ATP.
Bacteria using and generating arsenate
Some species of bacteriaBacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
obtain their energy by oxidizing
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
various fuels while reducing
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
arsenates to form arsenite
Arsenite
In chemistry an arsenite is a chemical compound containing an arsenic oxoanion where arsenic has oxidation state +3.The different forms of the anion are the next ones:* ortho-arsenite: AsO33-* meta-arsenite: AsO2-...
s. The enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s involved are known as arsenate reductases.
In 2008, bacteria were discovered that employ a version of photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
with arsenites as electron donor
Electron donor
An electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process....
s, producing arsenates (just like ordinary photosynthesis uses water as electron donor, producing molecular oxygen). The researchers conjectured that historically these photosynthesizing organisms produced the arsenates that allowed the arsenate-reducing bacteria to thrive.
In 2010, a team at NASA
NASA
The National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...
's Astrobiology Institute
NASA Astrobiology Institute
The NASA Astrobiology Institute was established in 1998 by the National Aeronautics and Space Administration "to develop the field of astrobiology and provide a scientific framework for flight missions". The NAI is a virtual, distributed organization that integrates astrobiology research and...
cultured samples of arsenic-resistant GFAJ-1
GFAJ-1
GFAJ-1 is a strain of rod-shaped bacterium in the family Halomonadaceae. The extremophile was isolated from the hypersaline and alkaline Mono Lake in eastern California by a research team led by NASA astrobiologist Felisa Wolfe-Simon...
bacteria from Mono Lake
Mono Lake
Mono Lake is a large, shallow saline lake in Mono County, California, formed at least 760,000 years ago as a terminal lake in a basin that has no outlet to the ocean...
, using a medium high in arsenate and low in phosphate concentration. The findings suggest that the bacteria may partially incorporate arsenate in place of phosphate in some biomolecules, including DNA, but the validity of this claim is still being debated.

