Asinger reaction
Encyclopedia
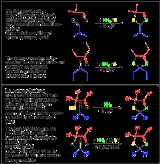
The Asinger-reaction was invented in 1956 by Friedrich Asinger
. The Asinger-reaction is a multicomponent reaction and is classified as A-4CR (short for Asinger-4 component reaction):
An α-halogenated carbonyl-component reacts with sodium-hydrogensulfide NaSH and forms in situ a Thiol
e. The thiole reacts directly with another carbonyl-component and ammonia
to form Thiazolines. The reaction works also by using sulphur, an in α–position substitutable ketone
, a further carbonyl-component and ammonia under formation of mixtures.
The formation of 3-Thiazolines also occurs by using α-thioaldehyde or α-thioketone
and ammonia.
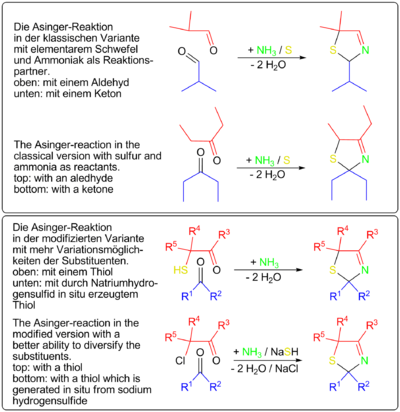 A simplyfied route of the Asinger-reaction was developed by Degussa. An α-halogenated carbonyl-compound reacts with sodium hydrogensulfide NaSH and creates in situ Thiol
A simplyfied route of the Asinger-reaction was developed by Degussa. An α-halogenated carbonyl-compound reacts with sodium hydrogensulfide NaSH and creates in situ Thiol
es, which reacts directly with aldehydes or ketones and ammonia to 3-thiazolines.. The chemical industry developed based on the Asinger-reaction multi stage processes for the production of pharmaceuticals like D-Penicillamine and the aminoacid DL-cysteine
.
Friedrich Asinger
Friedrich Asinger was a German chemist and professor for Technical Chemistry....
. The Asinger-reaction is a multicomponent reaction and is classified as A-4CR (short for Asinger-4 component reaction):
An α-halogenated carbonyl-component reacts with sodium-hydrogensulfide NaSH and forms in situ a Thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
e. The thiole reacts directly with another carbonyl-component and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
to form Thiazolines. The reaction works also by using sulphur, an in α–position substitutable ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
, a further carbonyl-component and ammonia under formation of mixtures.
The formation of 3-Thiazolines also occurs by using α-thioaldehyde or α-thioketone
Thioketone
Thioketones are organosulfur compounds related to conventional ketones. Instead of the formula R2C=O, thioketones have the formula R2C=S. Unhindered alkylthioketones are typically unstable; such compounds tend to form polymers or rings.-Preparative methods:Thiones are usually prepared from ketones...
and ammonia.

Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
es, which reacts directly with aldehydes or ketones and ammonia to 3-thiazolines.. The chemical industry developed based on the Asinger-reaction multi stage processes for the production of pharmaceuticals like D-Penicillamine and the aminoacid DL-cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
.

