Belite
Encyclopedia
Belite is an industrial mineral important in Portland cement
manufacture, a name for dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2 (C2S in cement chemist notation
).
The name was given by Törneborn in 1897 to a crystal identified in microscopic investigation of Portland Cement. Belite is a name in common use in the cement industry, and is not a recognised mineral name. Belite occurs naturally as the mineral larnite
.
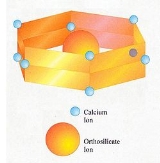
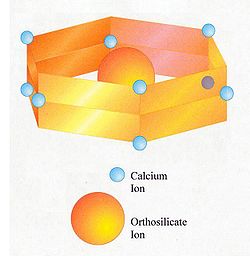 The belite found in Portland cement differs in composition from pure dicalcium silicate
The belite found in Portland cement differs in composition from pure dicalcium silicate
. It is a solid solution
and contains minor amounts of other oxide
s besides CaO and SiO2. A typical composition:
Based on this, the formula can be expressed as Ca1.94Mg0.02Na0.01K0.03Fe0.02Al0.07Si0.90P0.01O3.93. In practice, the composition varies with the bulk composition of the clinker
, subject to certain limits. Substitution of calcium ions or orthosilicate ions requires that electric charges be kept in balance. For instance, a limited number of orthosilicate (SiO44-) ions can be replaced with sulfate
(SO42-) ions, provided that for each sulfate ion, two aluminate
(AlO45-) ions are also substituted.
. This form does not hydrate, and is avoided in cement manufacture.
As the temperature rises, it passes through several polymorph
ic states:
. The other silicate, alite
contributes "early" strength, due to its higher reactivity. Belite reacts with water (roughly) to form calcium silicate hydrate
s (C-S-H) and portlandite
(Ca(OH)2) according to the reaction:
This rapid reaction is "chemically analogue" to the slow natural hydration of forsterite
(the magnesium end-member of olivine
) leading to the formation of serpentine
and brucite
in nature, although the kinetic of hydration of poorly crystallized artificial belite is much faster than the slow weathering of well crystallized Mg-olivine
under natural conditions.
The hydrate
phase, [3 CaO · 2 SiO2 · 3 H2O], is referred to as the "C-S-H
" phase. It grows as a mass of interlocking
needles that provide the strength of the hydrated cement system. Relatively high belite reactivity is desirable in Portland cement manufacture, and the formation of the unreactive γ-form must be rigorously avoided. This is achieved by rapid cooling, forming crystals that are small, distorted and highly defective. Defects provide sites for initial water attack. Failure to cool the clinker rapidly leads to inversion of belite to the γ-form. The γ-form has a substantially different structure and density, so that inversion leads to degradation of the crystal and its surrounding matrix, and can also trigger decomposition of the neightboring alite
. This is observed macroscopically as "dusting": the clinker nodule
s fall to a fine dust
.
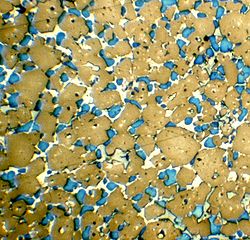 The minerals in Portland cement
The minerals in Portland cement
clinker may be observed and quantified by petrographic
microscopy. Clinker nodules are cut and ground to a flat, polished surface. The exposed minerals are made visible and identifiable by etching
the surface. The surface can then be observed in reflected light by optical microscopy. In the example, a clinker nodule has been polished and etched with hydrogen fluoride
vapour. The alite shows as brown, the belite as blue, and the melt phases as white. Electron microscopy can also be used, in which case the minerals may be identified by microprobe
analysis. The preferred method to quantify the minerals accurately is X-ray diffraction on the powdered clinker, using the Rietveld
analysis technique. Belite is much harder to grind in a cement mill
than alite.
Portland cement
Portland cement is the most common type of cement in general use around the world because it is a basic ingredient of concrete, mortar, stucco and most non-specialty grout...
manufacture, a name for dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2 (C2S in cement chemist notation
Cement chemist notation
Cement chemist notation was developed to simplify the formulas cement chemists use on a daily basis. It is a "short hand" way of writing the chemical formula of oxides of calcium, silicon, and various metals.-Abbreviations of oxides:...
).
The name was given by Törneborn in 1897 to a crystal identified in microscopic investigation of Portland Cement. Belite is a name in common use in the cement industry, and is not a recognised mineral name. Belite occurs naturally as the mineral larnite
Larnite
Larnite is a mineral, known as dicalcisum silicate.Dicalcium silicate is chemically, β–Ca2SiO4, sometimes represented by the formula 2CaO·SiO2.When used in the cement industry, the mineral is usually referred to as Belite....
.
Composition and structure

Calcium silicate
Calcium silicate is the chemical compound Ca2SiO4, also known as calcium orthosilicate and sometimes formulated 2CaO.SiO2. It is one of group of compounds obtained by reacting calcium oxide and silica in various ratios e.g. 3CaO.SiO2, Ca3SiO5; 2CaO.SiO2, Ca2SiO4; 3CaO.2SiO2, Ca3Si2O7 and...
. It is a solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
and contains minor amounts of other oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s besides CaO and SiO2. A typical composition:
| Oxide | Mass % |
|---|---|
| CaO | 63.5 |
| SiO2 | 31.5 |
| Al2O3 | 2.1 |
| Fe2O3 | 0.9 |
| MgO | 0.5 |
| SO3 | 0.1 |
| Na2O | 0.1 |
| K2O | 0.9 |
| TiO2 | 0.2 |
| P2O5 | 0.2 |
Based on this, the formula can be expressed as Ca1.94Mg0.02Na0.01K0.03Fe0.02Al0.07Si0.90P0.01O3.93. In practice, the composition varies with the bulk composition of the clinker
Clinker
Clinker may refer to:* Clinker , construction method for wooden boats* Clinker , waste from industrial processes* Clinker , a kilned then quenched cement product* Clinker brick, rough dark coloured bricks...
, subject to certain limits. Substitution of calcium ions or orthosilicate ions requires that electric charges be kept in balance. For instance, a limited number of orthosilicate (SiO44-) ions can be replaced with sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
(SO42-) ions, provided that for each sulfate ion, two aluminate
Aluminate
An aluminate is a compound containing aluminium and oxygen. When precipitated from solution, the anion condenses with more electropositive elements. The generally accepted form of the aluminate is a mononuclear tetrahedral complex that is negatively charged, Al4− or AlO2−...
(AlO45-) ions are also substituted.
Polymorphs
Dicalcium silicate is stable, and is readily prepared from reactive CaO and SiO2 at 300 °C. The low temperature form is γ-belite, or lime olivineOlivine
The mineral olivine is a magnesium iron silicate with the formula 2SiO4. It is a common mineral in the Earth's subsurface but weathers quickly on the surface....
. This form does not hydrate, and is avoided in cement manufacture.
As the temperature rises, it passes through several polymorph
Polymorphism (materials science)
Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
ic states:
| Temp°C | Name | Crystal |
|---|---|---|
| >1425 | α | Hexagonal |
| 1160-1425 | α'H | Monoclinic |
| 680-1160 | α'L | Monoclinic |
| 500-680 | β | Monoclinic |
| <500 | γ | Orthorhombic |
Hydration
Belite is the mineral in Portland cement responsible for development of "late" strengthStrength
- Physical ability :*Physical strength, as in people or animals*Superhuman strength, as in fictional characters*A common character attribute in role-playing gamesConflict between persons or groups:*Virtue and moral uprightness...
. The other silicate, alite
Alite
Alite is a name for tricalcium silicate, Ca3SiO5, sometimes formulated as 3CaO·SiO2 . It is the major, and characteristic, mineral phase in Portland cement. The name was given by Törneborn in 1897 to a crystal identified in microscopic investigation of Portland cement...
contributes "early" strength, due to its higher reactivity. Belite reacts with water (roughly) to form calcium silicate hydrate
Calcium silicate hydrate
Calcium Silicate Hydrate is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials.-Preparation:...
s (C-S-H) and portlandite
Portlandite
Portlandite is a rare oxide mineral, the naturally occurring form of calcium hydroxide . It is the calcium analogue of brucite .-Occurrence:...
(Ca(OH)2) according to the reaction:
- Belite + water → C-S-H phase + portlandite
- 2 Ca2SiO4 + 4 H2O → 3 CaO · 2 SiO2 · 3 H2O + Ca(OH)2
This rapid reaction is "chemically analogue" to the slow natural hydration of forsterite
Forsterite
Forsterite is the magnesium rich end-member of the olivine solid solution series. Forsterite crystallizes in the orthorhombic system with cell parameters a 4.75 Å , b 10.20 Å and c 5.98 Å .Forsterite is associated with igneous and metamorphic rocks and has also been found in meteorites...
(the magnesium end-member of olivine
Olivine
The mineral olivine is a magnesium iron silicate with the formula 2SiO4. It is a common mineral in the Earth's subsurface but weathers quickly on the surface....
) leading to the formation of serpentine
Serpentinite
Serpentinite is a rock composed of one or more serpentine group minerals. Minerals in this group are formed by serpentinization, a hydration and metamorphic transformation of ultramafic rock from the Earth's mantle...
and brucite
Brucite
Brucite is the mineral form of magnesium hydroxide, with the chemical formula Mg2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal vein mineral in metamorphosed limestones and chlorite schists; and formed during serpentinization of dunites...
in nature, although the kinetic of hydration of poorly crystallized artificial belite is much faster than the slow weathering of well crystallized Mg-olivine
Olivine
The mineral olivine is a magnesium iron silicate with the formula 2SiO4. It is a common mineral in the Earth's subsurface but weathers quickly on the surface....
under natural conditions.
- Forsterite + water → serpentine + brucite
- 2 Mg2SiO4 + 3 H2O → Mg3Si2O5(OH)4 + Mg(OH)2
The hydrate
Hydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
phase, [3 CaO · 2 SiO2 · 3 H2O], is referred to as the "C-S-H
Calcium silicate hydrate
Calcium Silicate Hydrate is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials.-Preparation:...
" phase. It grows as a mass of interlocking
Interlocking
In railway signalling, an interlocking is an arrangement of signal apparatus that prevents conflicting movements through an arrangement of tracks such as junctions or crossings. The signalling appliances and tracks are sometimes collectively referred to as an interlocking plant...
needles that provide the strength of the hydrated cement system. Relatively high belite reactivity is desirable in Portland cement manufacture, and the formation of the unreactive γ-form must be rigorously avoided. This is achieved by rapid cooling, forming crystals that are small, distorted and highly defective. Defects provide sites for initial water attack. Failure to cool the clinker rapidly leads to inversion of belite to the γ-form. The γ-form has a substantially different structure and density, so that inversion leads to degradation of the crystal and its surrounding matrix, and can also trigger decomposition of the neightboring alite
Alite
Alite is a name for tricalcium silicate, Ca3SiO5, sometimes formulated as 3CaO·SiO2 . It is the major, and characteristic, mineral phase in Portland cement. The name was given by Törneborn in 1897 to a crystal identified in microscopic investigation of Portland cement...
. This is observed macroscopically as "dusting": the clinker nodule
Nodule
Nodule may refer to:*Nodule , a small knobbly rock or mineral cluster, such as a manganese nodule*Nodule , a small aggregation of cells*Nodule , a lesion similar to a papule...
s fall to a fine dust
Dust
Dust consists of particles in the atmosphere that arise from various sources such as soil dust lifted up by wind , volcanic eruptions, and pollution...
.
Detection

Portland cement
Portland cement is the most common type of cement in general use around the world because it is a basic ingredient of concrete, mortar, stucco and most non-specialty grout...
clinker may be observed and quantified by petrographic
Petrography
Petrography is a branch of petrology that focuses on detailed descriptions of rocks. Someone who studies petrography is called a petrographer. The mineral content and the textural relationships within the rock are described in detail. Petrographic descriptions start with the field notes at the...
microscopy. Clinker nodules are cut and ground to a flat, polished surface. The exposed minerals are made visible and identifiable by etching
Etching
Etching is the process of using strong acid or mordant to cut into the unprotected parts of a metal surface to create a design in intaglio in the metal...
the surface. The surface can then be observed in reflected light by optical microscopy. In the example, a clinker nodule has been polished and etched with hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
vapour. The alite shows as brown, the belite as blue, and the melt phases as white. Electron microscopy can also be used, in which case the minerals may be identified by microprobe
Microprobe
A microprobe is an instrument that applies a stable and well-focused beam of charged particles to a sample.-Types:When the primary beam consists of accelerated electrons, the probe is termed an electron microprobe, when the primary beam consists of accelerated ions, the term Ion Microprobe is used...
analysis. The preferred method to quantify the minerals accurately is X-ray diffraction on the powdered clinker, using the Rietveld
Rietveld refinement
Rietveld refinement is a technique devised by Hugo Rietveld for use in the characterisation ofcrystalline materials. The neutron and x-ray diffractionof powder samples results in a pattern characterised by reflections at certain positions...
analysis technique. Belite is much harder to grind in a cement mill
Cement mill
A cement mill is the equipment used to grind the hard, nodular clinker from the cement kiln into the fine grey powder that is cement...
than alite.
See also
- Hydration reaction of forsterite (olivine) in serpentinisation
- CCNCement chemist notationCement chemist notation was developed to simplify the formulas cement chemists use on a daily basis. It is a "short hand" way of writing the chemical formula of oxides of calcium, silicon, and various metals.-Abbreviations of oxides:...
, cement chemist notation

