Solid solution
Encyclopedia
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
-state
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
of one or more solutes
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
in a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
. Such a mixture
Mixture
In chemistry, a mixture is a material system made up by two or more different substances which are mixed together but are not combined chemically...
is considered a solution rather than a compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
when the crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
. This often happens when the two elements (generally metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s) involved are close together on the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
; conversely, a chemical compound is generally a result of the non-proximity of the two metals involved on the periodic table.
Details
The solute may incorporate into the solvent crystal lattice substitutionSubstitution
Substitution may refer to:- Sciences :* Substitution , a syntactic transformation on strings of symbols of a formal language* Substitution of variables* Substitution cipher, a method of encryption...
ally, by replacing a solvent particle in the lattice, or interstitially
Interstitial defect
Interstitials are a variety of crystallographic defects, i.e. atoms which occupy a site in the crystal structure at which there is usually not an atom, or two or more atoms sharing one or more lattice sites such that the number of atoms is larger than the number of lattice sites.They are generally...
, by fitting into the space between solvent particles. Both of these types of solid solution affect the properties of the material by distorting the crystal lattice and disrupting the physical and electrical homogeneity of the solvent material.
Some mixtures will readily form solid solutions over a range of concentrations, while other mixtures will not form solid solutions at all. The propensity for any two substances to form a solid solution is a complicated matter involving the chemical
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, crystallographic
Crystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
, and quantum
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
properties of the substances in question. Solid solutions, in accordance with the Hume-Rothery rules
Hume-Rothery rules
The Hume-Rothery rules, named after William Hume-Rothery, are a set of basic rules describing the conditions under which an element could dissolve in a metal, forming a solid solution...
, may form if the solute and solvent have:
- Similar atomic radiiAtomic radiusThe atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons...
(15% or less difference) - SameCrystalliteCrystallites are small, often microscopic crystals that, held together through highly defective boundaries, constitute a polycrystalline solid. Metallurgists often refer to crystallites as grains.- Details :...
crystal structureCrystal structureIn mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry... - Similar electronegativitiesElectronegativityElectronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
- Similar valencyValence (chemistry)In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
The phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
in Fig. 1 displays an alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
of two metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
s which forms a solid solution at all relative concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
s of the two species. In this case, the pure phase of each element is of the same crystal structure, and the similar properties of the two elements allow for unbiased substitution through the full range of relative concentrations.
Solid solutions have important commercial and industrial applications, as such mixtures often have superior properties to pure materials. Many metal alloys are solid solutions. Even small amounts of solute can affect the electrical and physical properties of the solvent.
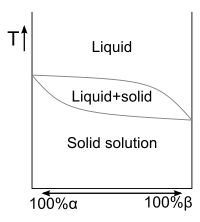
The binary phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
in Fig. 2 at right shows the phases of a mixture of two substances in varying concentrations,
 and
and  . The region labeled "
. The region labeled " " is a solid solution, with
" is a solid solution, with  acting as the solute in a matrix of
acting as the solute in a matrix of  . On the other end of the concentration scale, the region labeled "
. On the other end of the concentration scale, the region labeled " " is also a solid solution, with
" is also a solid solution, with  acting as the solute in a matrix of
acting as the solute in a matrix of  . The large solid region in between the
. The large solid region in between the  and
and  solid solutions, labeled "
solid solutions, labeled " and
and  ", is not a solid solution. Instead, an examination of the microstructure
", is not a solid solution. Instead, an examination of the microstructureMicrostructure
Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope above 25× magnification...
of a mixture in this range would reveal two phases — solid solution
 -in-
-in- and solid solution
and solid solution  -in-
-in- would form separate phases, perhaps lamella
would form separate phases, perhaps lamellaLamellar structure
Lamellar structures or microstructures are composed of fine, alternating layers of different materials in the form of lamellae. They are often observed in cases where a phase transformation front moves quickly, leaving behind two solid products, as in rapid cooling of eutectic or eutectoid ...
or grains
Crystallite
Crystallites are small, often microscopic crystals that, held together through highly defective boundaries, constitute a polycrystalline solid. Metallurgists often refer to crystallites as grains.- Details :...
.
Application
In the phase diagram, the unalloyed extreme left and right concentrations, and the dip in the center, the material will be solid and become liquid as heat is added, where at other proportions the material will enter a mushy or pasty phase. The mixture at dip point of the diagram is called a eutecticEutectic point
A eutectic system is a mixture of chemical compounds or elements that has a single chemical composition that solidifies at a lower temperature than any other composition. This composition is known as the eutectic composition and the temperature is known as the eutectic temperature...
alloy. Lead-tin mixtures formulated at that point (37/63 mixture) are useful when soldering electronic components, particularly if done manually, since the solid phase is quickly entered as the solder cools. In contrast, when lead-tin mixtures were used to solder seams in automobile bodies a pasty state enabled a shape to be formed with a wooden paddle or tool, so a 70-30 lead to tin ratio was used. (Lead is being removed from such applications owing to its toxicity and consequent difficulty in recycling devices and components that include lead.)
Exsolution
When a solid solution becomes unstable — due to a lower temperature, for example — exsolution occurs and the two phases separate into distinct microscopic to megascopic lamellae. This is mainly caused due to difference in cation size. Cations who have a large difference in radii are not likely to readily substitute.Take the alkali feldspar
Feldspar
Feldspars are a group of rock-forming tectosilicate minerals which make up as much as 60% of the Earth's crust....
minerals for example, whose end members are albite
Albite
Albite is a plagioclase feldspar mineral. It is the sodium endmember of the plagioclase solid solution series. As such it represents a plagioclase with less than 10% anorthite content. The pure albite endmember has the formula NaAlSi3O8. It is a tectosilicate. Its color is usually pure white, hence...
, NaAlSi3O8 and microcline
Microcline
Microcline is an important igneous rock-forming tectosilicate mineral. It is a potassium-rich alkali feldspar. Microcline typically contains minor amounts of sodium. It is common in granite and pegmatites. Microcline forms during slow cooling of orthoclase; it is more stable at lower temperatures...
, KAlSi3O8. At high temperatures Na+ and K+ readily substitute for each other and so the minerals will form a solid solution, yet at low temperatures albite
Albite
Albite is a plagioclase feldspar mineral. It is the sodium endmember of the plagioclase solid solution series. As such it represents a plagioclase with less than 10% anorthite content. The pure albite endmember has the formula NaAlSi3O8. It is a tectosilicate. Its color is usually pure white, hence...
can only substitute a small amount of K+ and the same applies for Na+ in the microcline
Microcline
Microcline is an important igneous rock-forming tectosilicate mineral. It is a potassium-rich alkali feldspar. Microcline typically contains minor amounts of sodium. It is common in granite and pegmatites. Microcline forms during slow cooling of orthoclase; it is more stable at lower temperatures...
, this leads to exsolution where they will separate into two separate phases. In the case of the alkali feldspar
Feldspar
Feldspars are a group of rock-forming tectosilicate minerals which make up as much as 60% of the Earth's crust....
minerals, thin white albite
Albite
Albite is a plagioclase feldspar mineral. It is the sodium endmember of the plagioclase solid solution series. As such it represents a plagioclase with less than 10% anorthite content. The pure albite endmember has the formula NaAlSi3O8. It is a tectosilicate. Its color is usually pure white, hence...
layers will alternate between typically pink microcline
Microcline
Microcline is an important igneous rock-forming tectosilicate mineral. It is a potassium-rich alkali feldspar. Microcline typically contains minor amounts of sodium. It is common in granite and pegmatites. Microcline forms during slow cooling of orthoclase; it is more stable at lower temperatures...
.

