Beryllium-10
Encyclopedia
Beryllium-10 is a radioactive isotope
of beryllium
. It is formed mainly by cosmic ray spallation
. Be-10 has a half-life
of 1.36 × 106 years, and decays by beta decay
to stable Boron-10
with a maximum energy of 556.2 keV.
Light elements in the atmosphere react with high energy galactic cosmic ray
particles. The spallation
of the reaction products is the source of 10Be (t, u particles like n or p):
 Because beryllium tends to exist in solution
Because beryllium tends to exist in solution
s below about pH
5.5 (and rainwater above many industrialized areas can have a pH less than 5), it will dissolve and be transported to the Earth's surface via rainwater. As the precipitation
quickly becomes more alkaline, beryllium drops out of solution. Cosmogenic 10Be thereby accumulates at the soil
surface, where its relatively long half-life
(1.387 million 12 years) permits a long residence time before decaying to 10B
. 10Be and its daughter product have been used to examine soil erosion, soil formation from regolith
, the development of lateritic soils
and the age of ice core
s. It is also formed in nuclear explosions by a reaction of fast neutrons with 13C in the carbon dioxide in air, and is one of the historical indicators of past activity at nuclear test sites.
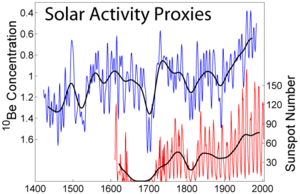
The rate of 10Be production depends on the strength of high energy galactic cosmic ray
s. Its influence is lower if the solar wind that is associated with solar activity is higher. The variation of 10Be concentration with time in Antarctic ice samples may be an indicator of solar activity in the past.
Isotopes of beryllium
Although beryllium has 12 known isotopes, only one of these isotopes is stable and a primordial nuclide. As such, it is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes are short-lived that none are primordial and their abundance is very low...
of beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
. It is formed mainly by cosmic ray spallation
Cosmic ray spallation
Cosmic ray spallation is a form of naturally occurring nuclear fission and nucleosynthesis. It refers to the formation of elements from the impact of cosmic rays on an object. Cosmic rays are highly energetic charged particles from outside of Earth ranging from protons, alpha particles, and nuclei...
. Be-10 has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 1.36 × 106 years, and decays by beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
to stable Boron-10
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
with a maximum energy of 556.2 keV.
Light elements in the atmosphere react with high energy galactic cosmic ray
Galactic cosmic ray
Galactic cosmic rays are cosmic rays that have their origin inside our Galaxy. GCRs are high-energy charged particles, and are usually protons, electrons, and fully ionized nuclei of light elements...
particles. The spallation
Spallation
In general, spallation is a process in which fragments of material are ejected from a body due to impact or stress. In the context of impact mechanics it describes ejection or vaporization of material from a target during impact by a projectile...
of the reaction products is the source of 10Be (t, u particles like n or p):
- 14N(t,5u)10Be; Example: 14N(n,p α)10Be
- 16O(t,7u)10Be

Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
s below about pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
5.5 (and rainwater above many industrialized areas can have a pH less than 5), it will dissolve and be transported to the Earth's surface via rainwater. As the precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
quickly becomes more alkaline, beryllium drops out of solution. Cosmogenic 10Be thereby accumulates at the soil
Soil
Soil is a natural body consisting of layers of mineral constituents of variable thicknesses, which differ from the parent materials in their morphological, physical, chemical, and mineralogical characteristics...
surface, where its relatively long half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
(1.387 million 12 years) permits a long residence time before decaying to 10B
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
. 10Be and its daughter product have been used to examine soil erosion, soil formation from regolith
Regolith
Regolith is a layer of loose, heterogeneous material covering solid rock. It includes dust, soil, broken rock, and other related materials and is present on Earth, the Moon, some asteroids, and other terrestrial planets and moons.-Etymology:...
, the development of lateritic soils
Laterite
Laterites are soil types rich in iron and aluminium, formed in hot and wet tropical areas. Nearly all laterites are rusty-red because of iron oxides. They develop by intensive and long-lasting weathering of the underlying parent rock...
and the age of ice core
Ice core
An ice core is a core sample that is typically removed from an ice sheet, most commonly from the polar ice caps of Antarctica, Greenland or from high mountain glaciers elsewhere. As the ice forms from the incremental build up of annual layers of snow, lower layers are older than upper, and an ice...
s. It is also formed in nuclear explosions by a reaction of fast neutrons with 13C in the carbon dioxide in air, and is one of the historical indicators of past activity at nuclear test sites.
The rate of 10Be production depends on the strength of high energy galactic cosmic ray
Galactic cosmic ray
Galactic cosmic rays are cosmic rays that have their origin inside our Galaxy. GCRs are high-energy charged particles, and are usually protons, electrons, and fully ionized nuclei of light elements...
s. Its influence is lower if the solar wind that is associated with solar activity is higher. The variation of 10Be concentration with time in Antarctic ice samples may be an indicator of solar activity in the past.

