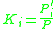Bubble point
Encyclopedia
When heating a liquid
consisting of two or more components, the bubble point is the point where first bubble of vapor
is formed. Given that vapor will probably have a different composition than the liquid, the bubble point (along with the dew point
) at different compositions are useful data when designing distillation systems.
For single component mixtures the bubble point and the dew point are the same and are referred to as the boiling point
.
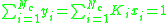
where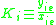 .
.
K is the distribution coefficient or K factor, defined as the ratio of mole fraction in the vapor phase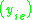 to the mole fraction in the liquid phase
to the mole fraction in the liquid phase 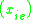 at equilibrium.
at equilibrium.
When Raoult's law and Dalton's law
hold for the mixture, the K factor is defined as the ratio of the vapor pressure to the total pressure of the system: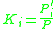
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
consisting of two or more components, the bubble point is the point where first bubble of vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
is formed. Given that vapor will probably have a different composition than the liquid, the bubble point (along with the dew point
Dew point
The dew point is the temperature to which a given parcel of humid air must be cooled, at constant barometric pressure, for water vapor to condense into liquid water. The condensed water is called dew when it forms on a solid surface. The dew point is a saturation temperature.The dew point is...
) at different compositions are useful data when designing distillation systems.
For single component mixtures the bubble point and the dew point are the same and are referred to as the boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
.
Calculating the bubble point
At the bubble point, the following relationship holds: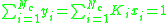
where
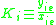 .
.K is the distribution coefficient or K factor, defined as the ratio of mole fraction in the vapor phase
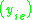 to the mole fraction in the liquid phase
to the mole fraction in the liquid phase 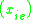 at equilibrium.
at equilibrium.When Raoult's law and Dalton's law
Dalton's law
In chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
hold for the mixture, the K factor is defined as the ratio of the vapor pressure to the total pressure of the system: