
Vapor
Encyclopedia

Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
phase at a temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
lower than its critical point.
This means that the vapor can be condensed
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
to a liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or to a solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
by increasing its pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
without reducing the temperature.
For example, water has a critical temperature of 374 °C (647 K), which is the highest temperature at which liquid water can exist. In the atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
at ordinary temperatures, therefore, gaseous water (known as water vapor
Water vapor
Water vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
) will condense to liquid if its partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
is increased sufficiently.
A vapor may co-exist with a liquid (or solid). When this is true, the two phases will be in equilibrium, and the gas pressure will equal the equilibrium vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
of the liquid (or solid).
Properties
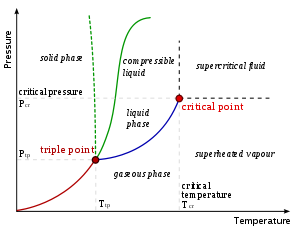
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
state, below the critical temperature of the substance. If the vapor is in contact with a liquid or solid phase, the two phases will be in a state of equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
. The term gas refers to a compressible fluid phase. Fixed gases are gases for which no liquid or solid can form at the temperature of the gas (such as air at typical ambient temperatures). A liquid or solid does not have to boil to release a vapor.
Vapor is responsible for the familiar processes of cloud
Cloud
A cloud is a visible mass of liquid droplets or frozen crystals made of water and/or various chemicals suspended in the atmosphere above the surface of a planetary body. They are also known as aerosols. Clouds in Earth's atmosphere are studied in the cloud physics branch of meteorology...
formation and condensation
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
. It is commonly employed to carry out the physical processes of distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
and headspace extraction
Headspace technology
Headspace technology is a technique developed in the 1980s to elucidate the odor compounds present in the air surrounding various objects. Usually the objects of interest are odoriferous objects such as plants, flowers and foods. Similar techniques are also used to analyze the interesting scents...
from a liquid sample prior to gas chromatography.
The constituent molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s of a vapor possess vibrational, rotational, and translational motion. These motions are considered in the kinetic theory of gases.
For example, water has a critical temperature of 374 °C (647 K), which is the highest temperature at which liquid water can exist.
Vapor pressure
The vapor pressureVapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
is the equilibrium pressure from a liquid or a solid at a specific temperature. The equilibrium vapor pressure of a liquid or solid is not affected by the amount of contact with the liquid or solid interface.
The normal boiling point of a liquid is the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
at which the vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
is equal to one atmosphere (unit)
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
.
For two-phase systems (e.g., two liquid phases), the vapor pressure of the system is the sum of the vapor pressures of the two liquids. In the absence of stronger inter-species attractions between like-like or like-unlike molecules, the vapor pressure follows Raoult's Law, which states that the partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of each component is the product of the vapor pressure of the pure component and its mole fraction in the mixture. The total vapor pressure is the sum of the component partial pressures.
The physical chemistry
Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
behind distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
is based on manipulating the equilibrium
Vapor-liquid equilibrium
Vapor–liquid equilibrium is a condition where a liquid and its vapor are in equilibrium with each other, a condition or state where the rate of evaporation equals the rate of condensation on a molecular level such that there is no net vapor-liquid interconversion...
occurring between the liquid and vapor phases of a molecule in solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
.
Examples

- PerfumePerfumePerfume is a mixture of fragrant essential oils and/or aroma compounds, fixatives, and solvents used to give the human body, animals, objects, and living spaces "a pleasant scent"...
s contain chemicals that vaporize at different temperatures and at different rate in scent accords known as noteNote (perfumery)Notes in perfumery are descriptors of scents that can be sensed upon the application of a perfume. Notes are separated into three classes; top/head notes, middle/heart notes, and base notes; which denote groups of smells that can be sensed with respect to the time after the application of a perfume...
s. - AtmosphericEarth's atmosphereThe atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
water vaporWater vaporWater vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
is found near the earth's surface, and may condense into small liquid droplets and form meteorological phenomena such as fogFogFog is a collection of water droplets or ice crystals suspended in the air at or near the Earth's surface. While fog is a type of stratus cloud, the term "fog" is typically distinguished from the more generic term "cloud" in that fog is low-lying, and the moisture in the fog is often generated...
, mistMistMist is a phenomenon of small droplets suspended in air. It can occur as part of natural weather or volcanic activity, and is common in cold air above warmer water, in exhaled air in the cold, and in a steam room of a sauna. It can also be created artificially with aerosol canisters if the...
and haarHaar (fog)In meteorology, haar is a coastal fog along certain lands bordering the North Sea; the term is primarily but not only, applied in eastern Scotland. Research has shown that haar is typically formed over the sea and is brought to land by wind advection....
. - Mercury-vapor lampMercury-vapor lampA mercury-vapor lamp is a gas discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger borosilicate glass bulb...
s and sodium vapor lampSodium vapor lampA sodium vapor lamp is a gas discharge lamp that uses sodium in an excited state to produce light. There are two varieties of such lamps: low pressure and high pressure...
s produce light from atoms in excited stateExcited stateExcitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
s.
Measuring vapor
Since it is in the gas phase, the amount of vapor present is quantified by the partial pressurePartial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of the gas. Also, vapors obey the barometric formula
Barometric formula
The barometric formula, sometimes called the exponential atmosphere or isothermal atmosphere, is a formula used to model how the pressure of the air changes with altitude.-Pressure equations:...
in a gravitational field just as conventional atmospheric gases do.
Vapors of flammable liquids
Flammable liquidFlammable liquid
Generally, a flammable liquid is a liquid that can catch fire.In the USA, there is a precise definition of flammable liquid as one with a flash point below 100 degrees Fahrenheit. Less-flammable liquids are defined as combustible liquids...
s do not burn when ignited. It is the vapor cloud above the liquid that will burn if the vapor's concentration is between the Lower Flammable Limit
Lower flammable limit
Lower flammability limit , usually expressed in volume per cent, is the lower end of the concentration range of a flammable solvent at a given temperature and pressure for which air/vapor mixtures can ignite. The flammability range is delineated by the upper and lower flammability limit. Outside...
(LFL) and Upper Flammable Limit (UFL) of the flammable liquid.
See also
- EvaporationEvaporationEvaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
- Water vaporWater vaporWater vapor or water vapour , also aqueous vapor, is the gas phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Under typical atmospheric conditions, water vapor is continuously...
- Dilution (equation)Dilution (equation)Dilution is a reduction in the concentration of a chemical . It is the process of reducing the concentration of a solute in solution, usually simply by mixing with more solvent. To dilute a solution means to add more solvent without the addition of more solute...
- Vapor pressureVapor pressureVapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
- Vapor Trail
- VaporizerVaporizerA vaporizer or vapouriser is a device used to extract for inhalation the active ingredients of plant material, commonly cannabis, tobacco, or other herbs or blends....
- Gas phase
- Henry's LawHenry's lawIn physics, Henry's law is one of the gas laws formulated by William Henry in 1803. It states that:An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid...
- Raoult's Law

