Bucherer reaction
Encyclopedia
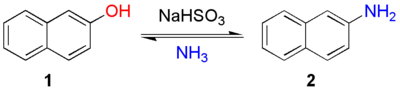
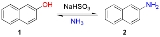
The Bucherer reaction in organic chemistry
is the reversible conversion of naphthol
to naphthylamine
in presence of ammonia
and sodium bisulfite
.
The French chemist Robert Lepetit was the first to discover the reaction in 1898 but it was the German chemist Hans Theodor Bucherer
(1869-1949) who discovered (independent from Lepetit) its reversibility and its potential in chemistry especially in industry. Bucherer published his results in 1904 and his name is connected to this reaction. The organic reaction
also goes by the name Bucherer-Lepetit reaction or (wrongly) the Bucherer-Le Petit reaction.
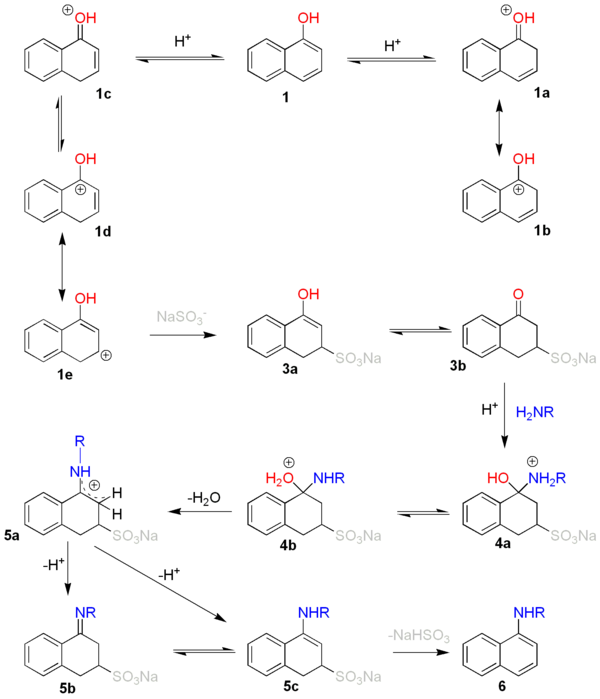
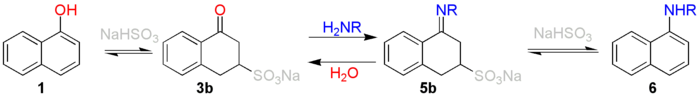
a proton
adds to a carbon atom with high electron density therefore by preference to C2 or C4 of naphthol
(1). This leads to resonance stabilized adducts 1a-1e.
De-aromatization of the first ring of the naphthalene
system occurs at the expense of 25 kcal/mol. In the next step a bisulfite
anion adds to C3 through 1e. This results in the formation of 3a which tautomer
izes to the more stable 3b to the sulfonic acid
of tetralone
. A nucleophilic addition
follows of the amine
with formation of 4a and its tautomer 4b loses water to form the resonance stabilized cation 5a. This compound is deprotonated to the imine
5b or the enamine
5c but an equilibrium exists between both species. The enamine eliminates sodium bisulfite with formation of naphthylamine 6.
It is important to stress that this is a reversible reaction
. The reaction is summarized as follows:
The Bucherer carbazole synthesis
is a related reaction.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is the reversible conversion of naphthol
Naphthol
Naphthol may refer to:* 1-Naphthol* 2-Naphthol...
to naphthylamine
Naphthylamine
Naphthylamine can refer to either of two isomeric chemical compounds:*1-Naphthylamine*2-Naphthylamine...
in presence of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
and sodium bisulfite
Sodium bisulfite
Sodium bisulfite is a chemical compound with the chemical formula NaHSO3. Sodium bisulfite is a food additive with E number E222. This salt of bisulfite can be prepared by bubbling sulfur dioxide in a solution of sodium carbonate in water...
.
The French chemist Robert Lepetit was the first to discover the reaction in 1898 but it was the German chemist Hans Theodor Bucherer
Hans Theodor Bucherer
Hans Theodor Bucherer was a German chemist and gave name to several chemical reactions, for example the Bucherer carbazole synthesis, the Bucherer reaction, and the Bucherer–Bergs reaction-Life:...
(1869-1949) who discovered (independent from Lepetit) its reversibility and its potential in chemistry especially in industry. Bucherer published his results in 1904 and his name is connected to this reaction. The organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
also goes by the name Bucherer-Lepetit reaction or (wrongly) the Bucherer-Le Petit reaction.
Mechanism
in the first step of the reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
adds to a carbon atom with high electron density therefore by preference to C2 or C4 of naphthol
Naphthol
Naphthol may refer to:* 1-Naphthol* 2-Naphthol...
(1). This leads to resonance stabilized adducts 1a-1e.
De-aromatization of the first ring of the naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
system occurs at the expense of 25 kcal/mol. In the next step a bisulfite
Bisulfite
Bisulfite ion is the ion HSO3−. Salts containing the HSO3− ion are termed bisulfites also known as sulfite lyes...
anion adds to C3 through 1e. This results in the formation of 3a which tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
izes to the more stable 3b to the sulfonic acid
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
of tetralone
Tetralone
Tetralone is an organic chemical compound with the molecular formula C10H10O. It is a common intermediate in organic synthesis. It is a ketone derivative of tetralin.The Haworth reaction is a classic method for the synthesis of tetralone...
. A nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
follows of the amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
with formation of 4a and its tautomer 4b loses water to form the resonance stabilized cation 5a. This compound is deprotonated to the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
5b or the enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
5c but an equilibrium exists between both species. The enamine eliminates sodium bisulfite with formation of naphthylamine 6.
It is important to stress that this is a reversible reaction
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
. The reaction is summarized as follows:
The Bucherer carbazole synthesis
Bucherer carbazole synthesis
The Bucherer carbazole synthesis is a chemical reaction used to synthesize carbazoles from naphthols and aryl hydrazines using sodium bisulfite. The reaction is named after Hans Theodor Bucherer.-References:...
is a related reaction.