Buffer gas
Encyclopedia
A buffer gas is an inert or nonflammable gas
. In the Earth's atmosphere
, nitrogen
acts as a buffer gas. A buffer gas adds pressure
to a system and controls the speed of combustion
with any oxygen
present. Any inert gas
such as helium
, neon
, or argon
will serve as a buffer gas.
transitions in alkali atoms
. A buffer gas usually consists of atomically inert gases such as helium
, argon
, and nitrogen
which are the primary gases used. Krypton
, neon
, and xenon
are also used, primarily for lighting. In most scenarios, buffer gases are used in conjunction with other molecules for the main purpose of causing collisions with the other co-existing molecules.
In fluorescent lamp
s, mercury
is used as the primary ion
from which light
is emitted. Krypton is the buffer gas used in conjunction with the mercury which is used to moderate the momentum of collisions of mercury ions in order to reduce the damage done to the electrode
s in the fluorescent lamp. Generally speaking, the longest lasting lamps are those with the heaviest noble gas
es as buffer gases.
Buffer gas loading techniques have been developed for use in cooling paramagnetic atoms
and molecules at ultra-cold temperatures. The buffer gas most commonly used in this sort of application is helium. Buffer gas cooling can be used on just about any molecule, as long as the molecule is capable of surviving multiple collisions with low energy helium atoms, which most molecules are capable of doing. Buffer gas cooling is allowing the molecules of interest to be cooled through elastic collision
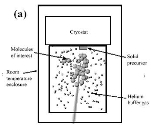
s with a cold buffer gas inside a chamber (see Figure (a)). If there are enough collisions between the buffer gas and the other molecules of interest before the molecules hit the walls of the chamber and are gone, the buffer gas will sufficiently cool the atoms. Of the two isotopes of helium (3He and 4He), the rarer 3He is sometimes used over 4He as it provides significantly higher vapor pressures and buffer gas density at sub-kelvin temperatures.
Buffer gases are also commonly used in compressors
used in power plants for supplying gas to gas turbines. The buffer gas fills the spaces between seal
s in the compressor. This space is usually about 2 micrometres wide. The gas must be completely dry and free of any contaminants. Contaminants can potentially lodge in the space between the seal and cause metal to metal contact in the compressor, leading to compressor failure (above right). In this case the buffer gas acts in a way much like oil does in an automotive engine’s bearings
.
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
. In the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
acts as a buffer gas. A buffer gas adds pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
to a system and controls the speed of combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
with any oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
present. Any inert gas
Inert gas
An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
such as helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
, or argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
will serve as a buffer gas.
Uses
Buffer gases are commonly used in many applications from high pressure discharge lamps to reduce line width of microwaveMicrowave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
transitions in alkali atoms
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
. A buffer gas usually consists of atomically inert gases such as helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
, and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
which are the primary gases used. Krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
, neon
Neon
Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or...
, and xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
are also used, primarily for lighting. In most scenarios, buffer gases are used in conjunction with other molecules for the main purpose of causing collisions with the other co-existing molecules.
In fluorescent lamp
Fluorescent lamp
A fluorescent lamp or fluorescent tube is a gas-discharge lamp that uses electricity to excite mercury vapor. The excited mercury atoms produce short-wave ultraviolet light that then causes a phosphor to fluoresce, producing visible light. A fluorescent lamp converts electrical power into useful...
s, mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
is used as the primary ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
from which light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
is emitted. Krypton is the buffer gas used in conjunction with the mercury which is used to moderate the momentum of collisions of mercury ions in order to reduce the damage done to the electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s in the fluorescent lamp. Generally speaking, the longest lasting lamps are those with the heaviest noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es as buffer gases.
Buffer gas loading techniques have been developed for use in cooling paramagnetic atoms
Paramagnetism
Paramagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
and molecules at ultra-cold temperatures. The buffer gas most commonly used in this sort of application is helium. Buffer gas cooling can be used on just about any molecule, as long as the molecule is capable of surviving multiple collisions with low energy helium atoms, which most molecules are capable of doing. Buffer gas cooling is allowing the molecules of interest to be cooled through elastic collision
Elastic collision
An elastic collision is an encounter between two bodies in which the total kinetic energy of the two bodies after the encounter is equal to their total kinetic energy before the encounter...
s with a cold buffer gas inside a chamber (see Figure (a)). If there are enough collisions between the buffer gas and the other molecules of interest before the molecules hit the walls of the chamber and are gone, the buffer gas will sufficiently cool the atoms. Of the two isotopes of helium (3He and 4He), the rarer 3He is sometimes used over 4He as it provides significantly higher vapor pressures and buffer gas density at sub-kelvin temperatures.
Buffer gases are also commonly used in compressors
Gas compressor
A gas compressor is a mechanical device that increases the pressure of a gas by reducing its volume.Compressors are similar to pumps: both increase the pressure on a fluid and both can transport the fluid through a pipe. As gases are compressible, the compressor also reduces the volume of a gas...
used in power plants for supplying gas to gas turbines. The buffer gas fills the spaces between seal
Seal (mechanical)
A mechanical seal is a device which helps join systems or mechanisms together by preventing leakage , containing pressure, or excluding contamination...
s in the compressor. This space is usually about 2 micrometres wide. The gas must be completely dry and free of any contaminants. Contaminants can potentially lodge in the space between the seal and cause metal to metal contact in the compressor, leading to compressor failure (above right). In this case the buffer gas acts in a way much like oil does in an automotive engine’s bearings
Bearing (mechanical)
A bearing is a device to allow constrained relative motion between two or more parts, typically rotation or linear movement. Bearings may be classified broadly according to the motions they allow and according to their principle of operation as well as by the directions of applied loads they can...
.

