CIDNP
Encyclopedia
CIDNP often pronounced like "kidnap", is a non-Boltzmann
nuclear spin
state distribution produced in thermal or photochemical reactions
, usually from colligation and diffusion, or disproportionation
of radical pairs, and detected by NMR spectroscopy
as enhanced absorption
or emission signals. CIDNP was discovered in 1967 by Bargon and Fischer, and, independently, by Ward and Lawler. Early theories were based on dynamic nuclear polarisation
(hence the name). The subsequent experiments, however, have found that in many cases DNP fails to explain CIDNP polarization phase
. In 1969 an alternative explanation was proposed by Closs, and, independently, by Kaptein and Oosterhoff, which relied on the ability of nuclear spin interactions to alter the recombination
probability in reactions that proceed through radical
pairs. This mechanism, known as the Radical Pair Mechanism is currently accepted as the most common cause of CIDNP. There are, however, exceptions, and the DNP mechanism was found to be operational, for example, in many fluorine-containing radicals.
, which excites the flavin mononucleotide
(FMN) photosensitizer to the singlet excited state. The fluorescence
quantum yield
of this state is rather low, and approximately half of the molecules undergo intersystem crossing
into the long-lived triplet state
. Triplet FMN has a remarkable electron affinity
. If a molecule with a low ionization potential
(e.g. phenol
s, polyaromatics
) is present in the system, the diffusion-limited electron transfer reaction forms a spin-correlated triplet electron transfer state – a radical pair. The actual kinetics
are rather complicated and may involve multiple (de)protonations
and hence exhibit pH
dependence.
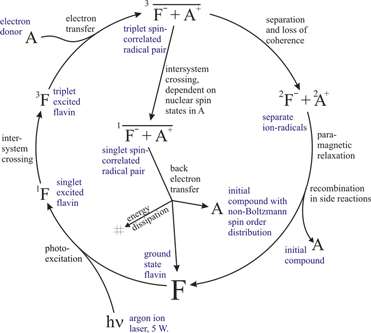 The radical pair may either cross over to a singlet electron state and then recombine, or separate and perish in side reactions. The relative probability of these two pathways for a given radical pair depends on the nuclear spin state and leads to the nuclear spin state sorting and observable nuclear polarization.
The radical pair may either cross over to a singlet electron state and then recombine, or separate and perish in side reactions. The relative probability of these two pathways for a given radical pair depends on the nuclear spin state and leads to the nuclear spin state sorting and observable nuclear polarization.
spectra of the reaction products, CIDNP has been exploited for the last 30 years to characterise transient free radicals and their reaction mechanism
s. In certain cases, CIDNP also offers the possibility of large improvements in NMR
sensitivity. The principal application of this photo-CIDNP technique, as devised by Kaptein in 1978, has been to protein
s in which the aromatic amino acid residues histidine
, tryptophan
and tyrosine
can be polarized using flavins or other aza-
aromatics as photosensitisers. The key feature of the method is that only solvent
accessible histidine
, tryptophan
and tyrosine
residues can undergo the radical pair reactions that result in nuclear polarization. Photo-CIDNP has thus been used to probe the surface structure of protein
s, both in native and partially folded
states, and their interactions with molecules that modify the accessibility of the reactive side chains.
Although usually observed in liquids, the photo-CIDNP effect has also been detected in solid state, for example on 13C and 15N nuclei in photosynthetic reaction centres, where significant nuclear polarization can accumulate as a result of spin selection processes in the electron transfer reactions.
Boltzmann distribution
In chemistry, physics, and mathematics, the Boltzmann distribution is a certain distribution function or probability measure for the distribution of the states of a system. It underpins the concept of the canonical ensemble, providing its underlying distribution...
nuclear spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
state distribution produced in thermal or photochemical reactions
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
, usually from colligation and diffusion, or disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
of radical pairs, and detected by NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
as enhanced absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
or emission signals. CIDNP was discovered in 1967 by Bargon and Fischer, and, independently, by Ward and Lawler. Early theories were based on dynamic nuclear polarisation
Dynamic nuclear polarisation
Dynamic nuclear polarization results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann...
(hence the name). The subsequent experiments, however, have found that in many cases DNP fails to explain CIDNP polarization phase
Phase (waves)
Phase in waves is the fraction of a wave cycle which has elapsed relative to an arbitrary point.-Formula:The phase of an oscillation or wave refers to a sinusoidal function such as the following:...
. In 1969 an alternative explanation was proposed by Closs, and, independently, by Kaptein and Oosterhoff, which relied on the ability of nuclear spin interactions to alter the recombination
Carrier generation and recombination
In the solid state physics of semiconductors, carrier generation and recombination are processes by which mobile charge carriers are created and eliminated. Carrier generation and recombination processes are fundamental to the operation of many optoelectronic semiconductor devices, such as...
probability in reactions that proceed through radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
pairs. This mechanism, known as the Radical Pair Mechanism is currently accepted as the most common cause of CIDNP. There are, however, exceptions, and the DNP mechanism was found to be operational, for example, in many fluorine-containing radicals.
Radical Pair Mechanism
The generation of CIDNP in a typical photochemical system (target + photosensitizer, flavin in this example) is a cyclic photochemical process shown schematically in Figure 1. The chain of reactions is initiated by a blue light photonPhoton
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
, which excites the flavin mononucleotide
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
(FMN) photosensitizer to the singlet excited state. The fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
quantum yield
Quantum yield
The quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
of this state is rather low, and approximately half of the molecules undergo intersystem crossing
Intersystem crossing
Intersystem crossing is a radiationless process involving a transition between two electronic states with different spin multiplicity.-Singlet and triplet states:...
into the long-lived triplet state
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
. Triplet FMN has a remarkable electron affinity
Electron affinity
The Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
. If a molecule with a low ionization potential
Ionization potential
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
(e.g. phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s, polyaromatics
Polycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbons , also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are potent atmospheric pollutants that consist of fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH...
) is present in the system, the diffusion-limited electron transfer reaction forms a spin-correlated triplet electron transfer state – a radical pair. The actual kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
are rather complicated and may involve multiple (de)protonations
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
and hence exhibit pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
dependence.

Applications
Detected as enhanced absorptive or emissive signals in the NMRNMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
spectra of the reaction products, CIDNP has been exploited for the last 30 years to characterise transient free radicals and their reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
s. In certain cases, CIDNP also offers the possibility of large improvements in NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
sensitivity. The principal application of this photo-CIDNP technique, as devised by Kaptein in 1978, has been to protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s in which the aromatic amino acid residues histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
, tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
and tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
can be polarized using flavins or other aza-
Aza-
The prefix aza- is used in organic chemistry to form names of organic compounds where a carbon atom is replaced by a nitrogen atom. Sometimes a number between hyphens is inserted before it to state which atom the nitrogen atom replaces...
aromatics as photosensitisers. The key feature of the method is that only solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
accessible histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
, tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
and tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
residues can undergo the radical pair reactions that result in nuclear polarization. Photo-CIDNP has thus been used to probe the surface structure of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, both in native and partially folded
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
states, and their interactions with molecules that modify the accessibility of the reactive side chains.
Although usually observed in liquids, the photo-CIDNP effect has also been detected in solid state, for example on 13C and 15N nuclei in photosynthetic reaction centres, where significant nuclear polarization can accumulate as a result of spin selection processes in the electron transfer reactions.

