
Carbohydrate conformation
Encyclopedia
Carbohydrate conformation is the characteristic 3-dimensional shape of a carbohydrate
. Conformations of monosaccharide
and oligosaccharide
heavily influence their reactivity and recognition by other molecules, which are essential to mammals and other organisms.
Lectins are an example of sugar binding proteins that are highly specific to the conformation of a particular carbohydrate. Specific carbohydrates serve as cell surface recognition signals for antibodies, hormones and toxins. Steric and stereoelectronic effects are common interactions that dictate the 3-dimensional shape of a carbohydrate.
and furanose
forms can exist in different conformers and one can interconvert between the different conformations if an energy penalty is met. For the furanose system there are two possible conformers: Twist (T) and Envelope (E). In the pyranose system four conformers are possible: Chair (C), Boat (B), Skew (S), Half-Chair (H) or Envelope (E). In all cases there are four or more atom
s that make up a plane. In order to define which atoms are above and below the plane one must orient the molecule so that the atoms are numbered clockwise
when looking form the top. Atoms above the plane are prefix
ed as a superscript and atoms below the plane are suffix
ed as a subscript. If the ring oxygen is above or below the plane it must be prefixed or suffixed appropriately
of 60° between adjacent substituents thus making it the most stable conformer. Since there are two possible chair conformation steric and stereoelectronic effects such as the anomeric effect
, 1,3 diaxial interactions, dipole
s and intramolecular hydrogen bonding must be taken into consideration when looking at relative energies. Conformations with 1,3 diaxial interactions are usually disfavored due to steric congestion and can shift equilibrium
to the other chair form (example: 1C4 to 4C1).
The size of the substituents greatly affects this equilibrium. However, intramolecular hydrogen bonding can be an example of a stabilizing 1,3 diaxial interaction. Dipoles also play a role in conformer stability, aligned dipoles lead to an increase in energy while opposed dipoles lead to a lowering of energy hence a stabilizing effect, this can be complicated by solvent effects. Polar solvents tend to stabilize aligned dipoles. All interaction must be taken into account when determining a preferred conformation
Conformations of five membered rings are limited to two, envelope and twist. The envelope conformation has four atoms in a plane while the twist form only has three. In the envelope form two different scenarios can be envisioned; one where the ring oxygen is in the four atom plane and one where it is puckered above or below the plane. When the ring oxygen is not in the plane the substituents eclipse and when it is in the plane torsional strain is relieved. Conformational analysis for the twist form is similar thus leading to the two forms being very close in energy.
s are diastereoisomers of glycoside
s, hemiacetal
s or related cyclic forms of sugars, or related molecules differing in configuration only at C-1. When the stereochemistry
of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
. The anomeric effect
more accurately called the endo-anomeric effect is the propensity for heteroatom
s at C-1 to be oriented axially. This is counter intuitive as one would expect the equatorially anomer to be the thermodynamic product. This effect has been rationalized through dipole-dipole repulsion and n-σ* arguments.
.
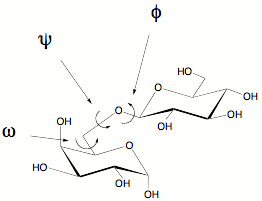 Three angles are described by φ, ψ and ω (in the case of glycosidic linkages via O-6). Steric considerations and anomeric effects need to be taken into consideration when looking at preferred angles.
Three angles are described by φ, ψ and ω (in the case of glycosidic linkages via O-6). Steric considerations and anomeric effects need to be taken into consideration when looking at preferred angles.
s exist in equilibrium
between their acyclic and cyclic forms with less than 1% in the acyclic form. The open chain form can close to give the pyranose
and furanose
with both the α and β anomers present for each. The equilibrium population of conformers depends on their relative energies which can be determined to a rough approximation using steric and stereoelectronic arguments. It has been shown that cations in solution can shift the equilibrium.
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
. Conformations of monosaccharide
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
and oligosaccharide
Oligosaccharide
An oligosaccharide is a saccharide polymer containing a small number of component sugars, also known as simple sugars...
heavily influence their reactivity and recognition by other molecules, which are essential to mammals and other organisms.
Lectins are an example of sugar binding proteins that are highly specific to the conformation of a particular carbohydrate. Specific carbohydrates serve as cell surface recognition signals for antibodies, hormones and toxins. Steric and stereoelectronic effects are common interactions that dictate the 3-dimensional shape of a carbohydrate.
Monosaccharide Conformation
PyranosePyranose
Pyranose is a collective term for carbohydrates that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds...
and furanose
Furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom...
forms can exist in different conformers and one can interconvert between the different conformations if an energy penalty is met. For the furanose system there are two possible conformers: Twist (T) and Envelope (E). In the pyranose system four conformers are possible: Chair (C), Boat (B), Skew (S), Half-Chair (H) or Envelope (E). In all cases there are four or more atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s that make up a plane. In order to define which atoms are above and below the plane one must orient the molecule so that the atoms are numbered clockwise
Clockwise
Circular motion can occur in two possible directions. A clockwise motion is one that proceeds in the same direction as a clock's hands: from the top to the right, then down and then to the left, and back to the top...
when looking form the top. Atoms above the plane are prefix
Prefix
A prefix is an affix which is placed before the root of a word. Particularly in the study of languages,a prefix is also called a preformative, because it alters the form of the words to which it is affixed.Examples of prefixes:...
ed as a superscript and atoms below the plane are suffix
Suffix
In linguistics, a suffix is an affix which is placed after the stem of a word. Common examples are case endings, which indicate the grammatical case of nouns or adjectives, and verb endings, which form the conjugation of verbs...
ed as a subscript. If the ring oxygen is above or below the plane it must be prefixed or suffixed appropriately
Conformational Analysis
Chair conformation of six membered rings have a dihedral angleDihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
of 60° between adjacent substituents thus making it the most stable conformer. Since there are two possible chair conformation steric and stereoelectronic effects such as the anomeric effect
Anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less hindered equatorial orientation that would...
, 1,3 diaxial interactions, dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
s and intramolecular hydrogen bonding must be taken into consideration when looking at relative energies. Conformations with 1,3 diaxial interactions are usually disfavored due to steric congestion and can shift equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
to the other chair form (example: 1C4 to 4C1).
The size of the substituents greatly affects this equilibrium. However, intramolecular hydrogen bonding can be an example of a stabilizing 1,3 diaxial interaction. Dipoles also play a role in conformer stability, aligned dipoles lead to an increase in energy while opposed dipoles lead to a lowering of energy hence a stabilizing effect, this can be complicated by solvent effects. Polar solvents tend to stabilize aligned dipoles. All interaction must be taken into account when determining a preferred conformation
Conformations of five membered rings are limited to two, envelope and twist. The envelope conformation has four atoms in a plane while the twist form only has three. In the envelope form two different scenarios can be envisioned; one where the ring oxygen is in the four atom plane and one where it is puckered above or below the plane. When the ring oxygen is not in the plane the substituents eclipse and when it is in the plane torsional strain is relieved. Conformational analysis for the twist form is similar thus leading to the two forms being very close in energy.
Anomers and related Effects
AnomerAnomer
In carbohydrate chemistry, an anomer is a special type of epimer. It is one of two stereoisomers of a cyclic saccharide that differs only in its configuration at the hemiacetal or hemiketal carbon, also called the anomeric carbon. Anomerization is the process of conversion of one anomer to the other...
s are diastereoisomers of glycoside
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to a non-carbohydrate moiety, usually a small organic molecule. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme...
s, hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
s or related cyclic forms of sugars, or related molecules differing in configuration only at C-1. When the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
Anomeric Effect
Anomers can be interconverted through a process known as mutarotationMutarotation
Mutarotation is the change in the optical rotation that occurs by epimerization...
. The anomeric effect
Anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less hindered equatorial orientation that would...
more accurately called the endo-anomeric effect is the propensity for heteroatom
Heteroatom
In organic chemistry, a heteroatom is any atom that is not carbon or hydrogen. Usually, the term is used to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure...
s at C-1 to be oriented axially. This is counter intuitive as one would expect the equatorially anomer to be the thermodynamic product. This effect has been rationalized through dipole-dipole repulsion and n-σ* arguments.
Reverse Anomeric Effect
The reverse anomeric effect, proposed in 1965 by R. U. Lemieux, is the tendency for electropositive groups at the anomeric position to be oriented equatorially. Original publication reported this phenomenon with N-(2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl)-4-methylpyridinium bromide. However, further studies have shown the effect to be a solvation and steric issue. It is accepted that there is no generalized reverse anomeric effect.Hydroxymethyl Conformation
Rotation around the C-5/C-6 bond is described by the angle ω. Three possible staggered conformations possible; gauche-trans (gt), gauche-gauche (gg), trans-gauche(tg). The name indicates the interaction between O-5 and OH-6 first followed by the interaction between OH-6 and C-4Oligosaccaharide Conformation
In addition to the factors affecting monosaccaride residues, conformational analysis of oligosaccharides and polysaccharides requires consideration additional factors.Exo-Anomeric Effect
Exo-anomeric effect is similar to the endo-anomeric effect. The difference being that the lone pair being donated is coming from the substituent at C-1. However, since the substituent can be either axial or equatorial there are two types of exo-anomeric effects, one from axial glycosides and one from equatorial glycosides as long as the donating orbital is anti-periplanar to the accepting orbitalMolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
.
Glycosidic Torsion Angles

Conformations in Solution
In solution, reducing monosaccharideMonosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s exist in equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
between their acyclic and cyclic forms with less than 1% in the acyclic form. The open chain form can close to give the pyranose
Pyranose
Pyranose is a collective term for carbohydrates that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds...
and furanose
Furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom...
with both the α and β anomers present for each. The equilibrium population of conformers depends on their relative energies which can be determined to a rough approximation using steric and stereoelectronic arguments. It has been shown that cations in solution can shift the equilibrium.
See also
- Anomeric effectAnomeric effectIn organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less hindered equatorial orientation that would...
- CarbohydrateCarbohydrateA carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
- PyranosePyranosePyranose is a collective term for carbohydrates that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds...
- FuranoseFuranoseA furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom...
- MonosaccharideMonosaccharideMonosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
- PolysaccharidePolysaccharidePolysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...

