
Charge number
Encyclopedia
Charge number or just valance of an ion
is the coefficient that, when multiplied by the elementary charge
, gives the ion's charge
.
For example, the charge on a chloride
ion, , is
, is 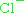 , where e is the elementary charge. This means the charge number for the ion is
, where e is the elementary charge. This means the charge number for the ion is 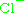 .
.
 is sometimes used as the symbol for the charge number. In that case, the charge of an ion could be written as
is sometimes used as the symbol for the charge number. In that case, the charge of an ion could be written as 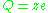 .
.
For an atomic nucleus
, which can be regarded as an ion having stripped off all electrons, the charge number is identical with the atomic number
Z (number of proton
s).
In particle physics
the charge number is a (derived) flavour quantum number, mostly denoted by Q (regarded as 'electric charge in units of e') rather than z. For color charge
d particles with like quark
s and hypothetical leptoquark
s the charge number is a broken multiple of 1/3.
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
is the coefficient that, when multiplied by the elementary charge
Elementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
, gives the ion's charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
.
For example, the charge on a chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
ion,
 , is
, is 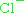 , where e is the elementary charge. This means the charge number for the ion is
, where e is the elementary charge. This means the charge number for the ion is 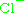 .
. is sometimes used as the symbol for the charge number. In that case, the charge of an ion could be written as
is sometimes used as the symbol for the charge number. In that case, the charge of an ion could be written as 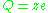 .
.For an atomic nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
, which can be regarded as an ion having stripped off all electrons, the charge number is identical with the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
Z (number of proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s).
In particle physics
Particle physics
Particle physics is a branch of physics that studies the existence and interactions of particles that are the constituents of what is usually referred to as matter or radiation. In current understanding, particles are excitations of quantum fields and interact following their dynamics...
the charge number is a (derived) flavour quantum number, mostly denoted by Q (regarded as 'electric charge in units of e') rather than z. For color charge
Color charge
In particle physics, color charge is a property of quarks and gluons that is related to the particles' strong interactions in the theory of quantum chromodynamics . Color charge has analogies with the notion of electric charge of particles, but because of the mathematical complications of QCD,...
d particles with like quark
Quark
A quark is an elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Due to a phenomenon known as color confinement, quarks are never directly...
s and hypothetical leptoquark
Leptoquark
Leptoquarks are hypothetical particles that carry information between quarks and leptons given a generation and allowing quarks and leptons to interact. They are color-triplet bosons that carry both lepton and baryon numbers. They are encountered in various extensions of the Standard Model, such as...
s the charge number is a broken multiple of 1/3.

