DeMayo reaction
Encyclopedia
The DeMayo reaction is a photochemical reaction in which the enol
of a 1,3-diketone
reacts with an alkene
(or another species with a C=C bond) and the resulting cyclobutane
ring undergoes a retro-aldol reaction
to yield a 1,5-diketone :
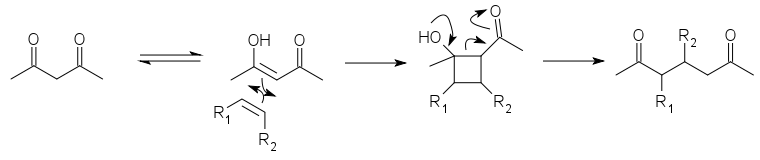
The net effect is to add the two carbon atoms in the C=C double bond between the two carbonyl groups of the diketone. It is thus useful in syntheses both as a relatively selective way to join two parts of a molecule and as a way to apply the more developed chemistry of 1,3-diketone synthesis to 1,5-diketones. The first part is a [2+2] cycloaddition
. The ensuing retro-aldol cleavage is favored by the relative instability of the cyclobutane ring.
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
of a 1,3-diketone
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
reacts with an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
(or another species with a C=C bond) and the resulting cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
ring undergoes a retro-aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
to yield a 1,5-diketone :

The net effect is to add the two carbon atoms in the C=C double bond between the two carbonyl groups of the diketone. It is thus useful in syntheses both as a relatively selective way to join two parts of a molecule and as a way to apply the more developed chemistry of 1,3-diketone synthesis to 1,5-diketones. The first part is a [2+2] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
. The ensuing retro-aldol cleavage is favored by the relative instability of the cyclobutane ring.

