
Decay scheme
Encyclopedia
The Decay scheme of a radioactive
substance is a graphical presentation of all the transitions occurring in a decay, and of their relationships.
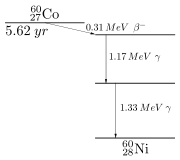 These relations can be quite complicated; a simple case is shown here: the decay scheme of the radioactive cobalt
These relations can be quite complicated; a simple case is shown here: the decay scheme of the radioactive cobalt
isotope
Cobalt-60
. 60Co decays by emitting an electron
(beta decay
) with a half-life
of 5.26 years into an excited state
of 60Ni, which then decays very fast to the ground state of 60Ni, via two gamma decays.
It is useful to think of the decay scheme as placed in a coordinate system, where the ordinate axis is energy, increasing from bottom to top, and the abscissa is the proton number, increasing from left to right. The arrows indicate the emitted particles. For the gamma rays (vertical arrows), the gamma energies are given; for the beta decay
(oblique arrow), the maximum beta energy.
Nickel is to the right of cobalt, since its proton number (28) is higher by one than that of cobalt (27). In beta decay, the proton number increases by one. For a positron decay and also for an alpha decay (see below), the oblique arrow would go from right to left since in these cases, the proton number decreases.
Since energy is conserved
and since the particles emitted carry away energy, arrows can only go downward (vertically or at an angle) in a decay scheme.
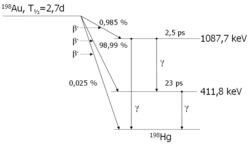 A somewhat more complicated scheme is shown here: the decay of the nuclide
A somewhat more complicated scheme is shown here: the decay of the nuclide
198Au which can be produced by irradiating natural gold in a nuclear reactor
. 198Au decays via beta decay
to one of two excited states or to the ground state of the mercury
isotope
198Hg. In the figure, mercury is to the right of gold, since the atomic number
of gold is 79, that of mercury is 80. The excited states decay after very short times (2.5 and 23 ps, resp.; 1 picosecond
is a millionth of a millionth of a second) to the ground state.
While excited nuclear states
are usually very short lived, decaying almost immediately after a beta decay (see above), the excited state of the technetium
isotope shown here to the right is comparatively long lived. It is therefore called "metastable
" (hence the "m" in 99mTc ). It decays to the ground state via gamma decay with a half-life of 6 hours.
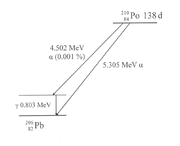 Here, to the left, we now have an alpha decay
Here, to the left, we now have an alpha decay
. It is the decay of the element Polonium
discovered by Marie Curie
, with mass number
210. The isotope
210Po is the penultimate member of the uranium-radium-decay series; it decays into a stable lead
-isotope with a half-life of 138 days. In almost all cases, the decay is via the emission of an alpha particle of 5.305 MeV. Only in one case of 100000, an alpha particle of lower energy appears; in this case, the decay leads to an excited level of 206Pb, which then decays to the ground state via gamma radiation.
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
substance is a graphical presentation of all the transitions occurring in a decay, and of their relationships.
Decay schemes of radioactive isotopes

Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
Cobalt-60
Cobalt-60
Cobalt-60, , is a synthetic radioactive isotope of cobalt. Due to its half-life of 5.27 years, is not found in nature. It is produced artificially by neutron activation of . decays by beta decay to the stable isotope nickel-60...
. 60Co decays by emitting an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
(beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
) with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 5.26 years into an excited state
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
of 60Ni, which then decays very fast to the ground state of 60Ni, via two gamma decays.
It is useful to think of the decay scheme as placed in a coordinate system, where the ordinate axis is energy, increasing from bottom to top, and the abscissa is the proton number, increasing from left to right. The arrows indicate the emitted particles. For the gamma rays (vertical arrows), the gamma energies are given; for the beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
(oblique arrow), the maximum beta energy.
Nickel is to the right of cobalt, since its proton number (28) is higher by one than that of cobalt (27). In beta decay, the proton number increases by one. For a positron decay and also for an alpha decay (see below), the oblique arrow would go from right to left since in these cases, the proton number decreases.
Since energy is conserved
Conservation law
In physics, a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves....
and since the particles emitted carry away energy, arrows can only go downward (vertically or at an angle) in a decay scheme.

Nuclide
A nuclide is an atomic species characterized by the specific constitution of its nucleus, i.e., by its number of protons Z, its number of neutrons N, and its nuclear energy state....
198Au which can be produced by irradiating natural gold in a nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
. 198Au decays via beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
to one of two excited states or to the ground state of the mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
198Hg. In the figure, mercury is to the right of gold, since the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
of gold is 79, that of mercury is 80. The excited states decay after very short times (2.5 and 23 ps, resp.; 1 picosecond
Picosecond
A picosecond is 10−12 of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 001 seconds. A picosecond is to one second as one second is to 31,700 years....
is a millionth of a millionth of a second) to the ground state.
While excited nuclear states
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
are usually very short lived, decaying almost immediately after a beta decay (see above), the excited state of the technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
isotope shown here to the right is comparatively long lived. It is therefore called "metastable
Metastability
Metastability describes the extended duration of certain equilibria acquired by complex systems when leaving their most stable state after an external action....
" (hence the "m" in 99mTc ). It decays to the ground state via gamma decay with a half-life of 6 hours.

Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
. It is the decay of the element Polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
discovered by Marie Curie
Marie Curie
Marie Skłodowska-Curie was a physicist and chemist famous for her pioneering research on radioactivity. She was the first person honored with two Nobel Prizes—in physics and chemistry...
, with mass number
Mass number
The mass number , also called atomic mass number or nucleon number, is the total number of protons and neutrons in an atomic nucleus. Because protons and neutrons both are baryons, the mass number A is identical with the baryon number B as of the nucleus as of the whole atom or ion...
210. The isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
210Po is the penultimate member of the uranium-radium-decay series; it decays into a stable lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
-isotope with a half-life of 138 days. In almost all cases, the decay is via the emission of an alpha particle of 5.305 MeV. Only in one case of 100000, an alpha particle of lower energy appears; in this case, the decay leads to an excited level of 206Pb, which then decays to the ground state via gamma radiation.

