
Detonation nanodiamond
Encyclopedia
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
that originates from a detonation
Detonation
Detonation involves a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations are observed in both conventional solid and liquid explosives, as well as in reactive gases...
. When an oxygen-deficient explosive mixture of TNT/RDX is detonated in a closed chamber, diamond particles with a diameter of ca. 5 nm are formed at the front of detonation wave in time of several microseconds.
History
Detonation nanodiamonds were first synthesized in 1962 by a group of Soviet scientists at VNIITF led by Yevgeny ZababakhinYevgeny Zababakhin
Yevgeny Ivanovich Zababakhin , January 16, 1917, Moscow, USSR — December 27, 1984) was a Soviet physicist, one of the chief designers of nuclear weapons in USSR, academician of the USSR Academy of Sciences, Eng. Lt. Gen. of Soviet Air Force...
, including K. V. Volkov, Vyacheslav Danilenko
Vyacheslav Danilenko
Dr. Vyacheslav Danilenko is a Ukrainian-born, former Soviet scientist who specializes in nanodiamonds.-Soviet Union:During the Soviet-era, he was employed at the nuclear installation known as NII-1011 located in the closed city of Chelyabinsk-70...
, and V. I. Elina.
Properties
The diamond yield after detonation crucially depends on the synthesis condition and especially on the heat capacity of the cooling medium in the detonation chamber (water, air, CO2, etc.). The higher the cooling capacity, the larger the diamond yield, which can reach 90%. After the synthesis, diamond is extracted from the soot using high-temperature high-pressure (autoclaveAutoclave
An autoclave is an instrument used to sterilize equipment and supplies by subjecting them to high pressure saturated steam at 121 °C for around 15–20 minutes depending on the size of the load and the contents. It was invented by Charles Chamberland in 1879, although a precursor known as the...
) boiling in acid for a long period (ca. 1–2 days). The boiling removes most of the metal contamination, originating from the chamber materials, and non-diamond carbon.
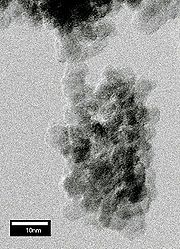
Various measurements, including X-ray diffraction and high-resolution transmission electron microscopy revealed that the size of the diamond grains in the soot is distributed around 5 nm. The grains are unstable with respect to aggregation and spontaneously form micrometre-sized clusters (see figure above). The adhesion is strong and contacts between a few nano-grains can hold a micrometre-sized cluster attached to a substrate.
Nanosized diamond has extremely large relative surface area. As a result, its surface spontaneously attaches water and hydrocarbon molecules from the ambient atmosphere. However, clean nanodiamond surface can be obtained with appropriate handling.
The detonation nanodiamond grains mostly have diamond cubic
Diamond cubic
The diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group IV also adopt this structure, including tin, the semiconductors silicon and germanium, and silicon/germanium...
lattice and are structurally imperfect. The major defects are multiple twins
Crystal twinning
Crystal twinning occurs when two separate crystals share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals in a variety of specific configurations. A twin boundary or composition surface separates the two crystals....
, as suggested by High-resolution transmission electron microscopy. Despite the carbon source for the diamond synthesis - TNT/RDX explosive mixture - being rich in nitrogen, concentration of paramagnetic nitrogen inside diamond grains is below 1 part per million (ppm). Paramagnetic nitrogen (neutral nitrogen atoms substituting carbon in the diamond lattice) is the major form of nitrogen in diamond, and thus the nitrogen content in DND is probably very low.
Alternative synthesis methods
Diamond nanocrystals can also be synthesized from a suspension of graphite in organic liquid at atmospheric pressure and room temperature using ultrasonic cavitation. The yield is approximately 10%. The cost of nanodiamonds produced by this method are estimated to be competitive with the HPHT process.An alternative synthesis technique is irradiation of graphite by high-energy laser pulses. The structure and particle size of the obtained diamond is rather similar to that obtained in explosion. In particular, many particles exhibit multiple twinning.
Applications
Commercial products based on nanodiamonds are available for the following applications:- LappingLappingLapping is a machining operation, in which two surfaces are rubbed together with an abrasive between them, by hand movement or by way of a machine.This can take two forms...
and polishing (e.g. Sufipol); - Additives to engine oils (e.g. ADDO);
- Dry lubricants for metal industry (Drawing of W-, Mo-, V-, Rh-wires);
- Reinforcing fillers for plastics and rubbers;
- Additives to galvanic electrolytes (e.g. DiamoSilb).
Use in medicine
Prof. Dean Ho, Dr. Houjin Huang, and Dr. Erik Pierstorff at Northwestern University, in collaboration with Dr. Eiji OsawaEiji Osawa
, a professor of computational chemistry, is famous for the prediction in 1970 of the C60 molecule.Osawa received his Master's of Engineering in chemistry from Kyoto University's Department of Industrial Chemistry and then became an engineer at Teijin Co., Ltd. In 1964, he returned to Kyoto...
at The NanoCarbon Research Institute in Japan, have demonstrated that nanomaterials can shuttle chemotherapy drugs to cells without producing the negative effects of today's delivery agents.
Clusters of the nanodiamonds surround the drugs ensuring that they remain separated from healthy cells, preventing unnecessary damage; upon reaching the intended targets, the drugs are released into the cancer cells. The leftover diamonds, hundreds of thousands of which could fit into the eye of a needle, do not induce inflammation in cells once they have done their job.
External links
- http://pubs.acs.org/cgi-bin/sample.cgi/jpcbfk/asap/pdf/jp066387v.pdf
- http://www.udayton.edu/News/Article/?contentId=2234
- http://research.ncl.ac.uk/nanoscale/research/nanodiamond.html Nanodiamond research at Newcastle University
- http://www.ioffe.rssi.ru/nanodiamond/ Ioffe Physico-Technical Institute of the Russian Academy
- http://www.cnn.com/2007/TECH/science/10/19/nanodiamonds.drugs/index.html

