
Diamagnetism
Encyclopedia

Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
in opposition to an externally applied magnetic field, thus causing a repulsive effect. Specifically, an external magnetic field alters the orbital velocity of electrons around their nuclei, thus changing the magnetic dipole moment. According to Lenz's law
Lenz's law
Lenz's law is a common way of understanding how electromagnetic circuits must always obey Newton's third law and The Law of Conservation of Energy...
, these electrons will oppose the magnetic field changes provided by the applied field, preventing them from building up. The result is that lines of magnetic flux
Magnetic flux
Magnetic flux , is a measure of the amount of magnetic B field passing through a given surface . The SI unit of magnetic flux is the weber...
curve away from the material. In most materials, diamagnetism is a weak effect, but under conditions of superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
, which only some materials may obtain, a strong quantum
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
effect can emerge wherein the lines are completely blocked, excluding a very thin layer at the material's surface. Diamagnets are materials with a magnetic permeability
Permeability (electromagnetism)
In electromagnetism, permeability is the measure of the ability of a material to support the formation of a magnetic field within itself. In other words, it is the degree of magnetization that a material obtains in response to an applied magnetic field. Magnetic permeability is typically...
less than
 (a relative permeability less than 1).
(a relative permeability less than 1).History
In 1778, Sebald Justinus BrugmansSebald Justinus Brugmans
Sebald Justinus Brugmans was a Dutch botanist, physician and professor of natural sciences.Brugmans studied at the University of Groningen, where he earned doctorates in medicine and philosophy, and also a masters degree in liberal arts...
was the first individual to observe that bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
and antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
were repelled by magnetic fields. However, the term diamagnetism was coined by Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
in September 1845, when he realized that all materials in nature possessed some form of diamagnetic response to an applied magnetic field.
Diamagnetic materials
| Material | χv (10−5) |
|---|---|
| Superconductor | −105 |
| Pyrolytic carbon Pyrolytic carbon Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production.... |
−40.0 |
| Bismuth Bismuth Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead... |
−16.6 |
| Mercury Mercury (element) Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum... |
−2.9 |
| Silver Silver Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal... |
−2.6 |
| Carbon (diamond) Diamond In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions... |
−2.1 |
| Lead Lead Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed... |
−1.8 |
| Carbon (graphite) Graphite The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal... |
−1.6 |
| Copper Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... |
−1.0 |
| Water Water Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a... |
−0.91 |
Diamagnetism is a very general phenomenon, because all electrons, including the electrons of an atom, will always make a weak contribution to the material's response. However, for materials that show some other form of magnetism (such as ferromagnetism
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
or paramagnetism
Paramagnetism
Paramagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
), the diamagnetism is completely overpowered. Substances that mostly display diamagnetic behaviour are termed diamagnetic materials, or diamagnets. Materials that are said to be diamagnetic are those that are usually considered by non-physicists to be non-magnetic, and include water
Water (properties)
Water is the most abundant compound on Earth's surface, covering about 70%. In nature, it exists in liquid, solid, and gaseous states. It is in dynamic equilibrium between the liquid and gas states at standard temperature and pressure. At room temperature, it is a tasteless and odorless liquid,...
, wood
Wood
Wood is a hard, fibrous tissue found in many trees. It has been used for hundreds of thousands of years for both fuel and as a construction material. It is an organic material, a natural composite of cellulose fibers embedded in a matrix of lignin which resists compression...
, most organic compounds such as petroleum and some plastics, and many metals including copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, particularly the heavy ones with many core electrons, such as mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
and bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
. The magnetic susceptibility of various molecular fragments are called Pascal's constants
Pascal's constants
Pascals’ constants are numbers used in the evaluation of the magnetic susceptibilities of coordination compounds. The magnetic susceptibility of a compound is the sum of the paramagnetic susceptibility associated with the unpaired electrons and the opposing diamagnetic susceptibility associated...
.
Diamagnetic materials have a relative magnetic permeability that is less than or equal to 1, and therefore a magnetic susceptibility
Magnetic susceptibility
In electromagnetism, the magnetic susceptibility \chi_m is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field...
which is less than 0 since susceptibility is defined as χv = μv − 1. This means that diamagnetic materials are repelled by magnetic fields. However, since diamagnetism is such a weak property its effects are not observable in everyday life. For example, the magnetic susceptibility of diamagnets such as water is χv = −9.05×10−6. The most strongly diamagnetic material is bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, χv = −1.66×10−4, although pyrolytic carbon
Pyrolytic carbon
Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production....
may have a susceptibility of χv = −4.00×10−4 in one plane. Nevertheless, these values are orders of magnitudes smaller than the magnetism exhibited by paramagnets and ferromagnets. Note that because χv is derived from the ratio of the internal magnetic field to the applied field, it is a dimensionless value.
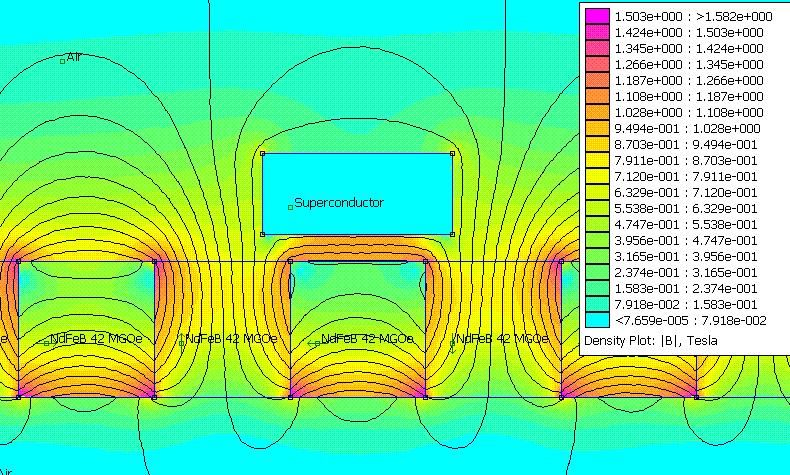
Meissner effect
The Meissner effect is the expulsion of a magnetic field from a superconductor during its transition to the superconducting state. The German physicists Walther Meissner and Robert Ochsenfeld discovered the phenomenon in 1933 by measuring the magnetic field distribution outside superconducting tin...
. However this effect is not due to eddy currents, as in ordinary diamagnetic materials (see the article on superconductivity
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
).
Additionally, all conductors exhibit an effective diamagnetism when they experience a changing magnetic field. The Lorentz force
Lorentz force
In physics, the Lorentz force is the force on a point charge due to electromagnetic fields. It is given by the following equation in terms of the electric and magnetic fields:...
on electrons causes them to circulate around forming eddy currents. The eddy currents then produce an induced magnetic field which opposes the applied field, resisting the conductor's motion.
Curving water surfaces
If a powerful magnet (such as a supermagnet) is covered with a layer of water (that is thin compared to the diameter of the magnet) then the field of the magnet significantly repels the water. This causes a slight dimple in the water's surface that may be seen by its reflection.Diamagnetic levitation

Earnshaw's theorem
Earnshaw's theorem states that a collection of point charges cannot be maintained in a stable stationary equilibrium configuration solely by the electrostatic interaction of the charges. This was first proven by British mathematician Samuel Earnshaw in 1842. It is usually referenced to magnetic...
seems to preclude the possibility of static magnetic levitation. However, Earnshaw's theorem only applies to objects with positive moments, such as ferromagnets (which have a permanent positive moment) and paramagnets (which induce a positive moment). These are attracted to field maxima, which do not exist in free space. Diamagnets (which induce a negative moment) are attracted to field minima, and there can be a field minimum in free space.
A thin slice of pyrolytic graphite, which is an unusually strong diamagnetic material, can be stably floated in a magnetic field, such as that from rare earth
Rare-earth magnet
Rare-earth magnets are strong permanent magnets made from alloys of rare earth elements. Developed in the 1970s and 80s, rare-earth magnets are the strongest type of permanent magnets made and have significant performance advantages over ferrite or alnico magnets...
permanent magnets. This can be done with all components at room temperature, making a visually effective demonstration of diamagnetism.
The Radboud University Nijmegen
Radboud University Nijmegen
Radboud University Nijmegen is a public university with a strong focus on research in Nijmegen, the Netherlands...
, the Netherlands
Netherlands
The Netherlands is a constituent country of the Kingdom of the Netherlands, located mainly in North-West Europe and with several islands in the Caribbean. Mainland Netherlands borders the North Sea to the north and west, Belgium to the south, and Germany to the east, and shares maritime borders...
, has conducted experiments where water and other substances were successfully levitated. Most spectacularly, a live frog (see figure) was levitated.
In September 2009, NASA's Jet Propulsion Laboratory in Pasadena, California announced they had successfully levitated mice using a superconducting magnet
Superconducting magnet
A superconducting magnet is an electromagnet made from coils of superconducting wire. They must be cooled to cryogenic temperatures during operation. In its superconducting state the wire can conduct much larger electric currents than ordinary wire, creating intense magnetic fields...
, an important step forward since mice are closer biologically to humans than frogs. They hope to perform experiments regarding the effects of microgravity on bone and muscle mass.
Recent experiments studying the growth of protein crystals has led to a technique using powerful magnets to allow growth in ways that counteract Earth's gravity.
A simple homemade device for demonstration can be constructed out of bismuth plates and a few permanent magnets that will levitate a permanent magnet.
Theory of diamagnetism
The Bohr–van Leeuwen theoremBohr–van Leeuwen theorem
The Bohr–van Leeuwen theorem is a theorem in the field of statistical mechanics. The theorem shows that when statistical mechanics and classical mechanics are applied consistently, the thermal average of the magnetization is always zero...
proves that there cannot be any diamagnetism or paramagnetism in a purely classical system. Yet the classical theory for Langevin diamagnetism gives the same prediction as the quantum theory. The classical theory is given below.
Langevin diamagnetism
The Langevin theory of diamagnetism applies to materials containing atoms with closed shells (see dielectrics). A field with intensity , applied to an electron
, applied to an electronElectron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
with charge
 and mass
and mass  , gives rise to Larmor precession
, gives rise to Larmor precessionLarmor precession
In physics, Larmor precession is the precession of the magnetic moments of electrons, atomic nuclei, and atoms about an external magnetic field...
with frequency
 . The number of revolutions per unit time is
. The number of revolutions per unit time is  , so the current for an atom with
, so the current for an atom with  electrons is (in SI units)
electrons is (in SI units)
The magnetic moment
Magnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
of a current loop is equal to the current times the area of the loop. Suppose the field is aligned with the
 axis. The average loop area can be given as
axis. The average loop area can be given as  , where
, where  is the mean square distance of the electrons perpendicular to the
is the mean square distance of the electrons perpendicular to the  axis. The magnetic moment
axis. The magnetic momentMagnetic moment
The magnetic moment of a magnet is a quantity that determines the force that the magnet can exert on electric currents and the torque that a magnetic field will exert on it...
is therefore

If the distribution of charge is spherically symmetric, we can suppose that the distribution of
 coordinates are independent and identically distributed. Then
coordinates are independent and identically distributed. Then  , where
, where  is the mean square distance of the electrons from the nucleus. Therefore
is the mean square distance of the electrons from the nucleus. Therefore  . If
. If  is the number of atoms per unit volume, the diamagnetic susceptibility
is the number of atoms per unit volume, the diamagnetic susceptibilityMagnetic susceptibility
In electromagnetism, the magnetic susceptibility \chi_m is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field...
is

Diamagnetism in metals
The Langevin theory does not apply to metals because they have non-localized electrons. The theory for the diamagnetism of a free electron gas is called Landau diamagnetism, and instead considers the weak counter-acting field that forms when their trajectories are curved due to the Lorentz forceLorentz force
In physics, the Lorentz force is the force on a point charge due to electromagnetic fields. It is given by the following equation in terms of the electric and magnetic fields:...
. Landau diamagnetism, however, should be contrasted with Pauli paramagnetism, an effect associated with the polarization of delocalized electrons' spins.
External links
- Video of a museum-style magnetic elevation train model which makes use of diamagnetism
- Videos of frogs and other diamagnets levitated in a strong magnetic field
- Video of levitating pyrolytic graphite
- Video of Meissner-Ochsenfeld effect involving liquid nitrogen
- Video of a piece of neodymium magnet levitating between blocks of bismuth.

