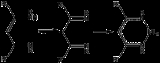
Diketimine
Encyclopedia
Diketimines or diimines are a family of ligands and ligand precursors derived from 1,2- and 1,3-diketone
s by replacement of the carbonyl oxygen atoms with NR groups, where R = aryl
, alkyl. Two families of diketimines are important in coordination chemistry and catalysis
: 1,2-diketimines and 1,3-diketimines.
s that are used to convert aldehydes and ketones to imine
s, Schiff base
s, and oxime
s. For example, acetylacetone
(2,4-pentanedione) and a primary alkyl- or arylamine will react, typically in acidified ethanol, to form a diketimine. 1,3-Diketimines derived from bulky amines, e.g. 2,6-disubstituted aniline
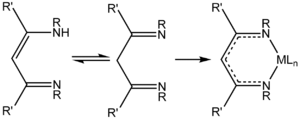
s, require prolonged reaction times. 1,3-Diketimines are often referred to as "HNacNac," a modification of the abbreviation Hacac used for α,β-diketones. These species can exist as a mixture of two tautomers.
as well as oxidized derivatives of o-phenylenediamine
. The steric properties of the substituents on nitrogen provide a means to control the axial coordination sites on a square planar
complex. Large planar substituents such as mesityl tend to be orthogonal to the MN2 plane. In this way, the axial coordination sites on a square planar complex are shielded. Such steric control is not possible for complexes of the related to 2,2'-bipyridine
, glyoximate
, and 9,10-phenanthroline
ligands.
 Deprotonation
Deprotonation
of HNacNac compounds affords anionic bidentate ligands that form a variety of coordination complexes. Some derivatives with large R group
s can been used to stabilize low valent main group
and transition metal
complexes. Unlike the situation for the acetylacetonates, the steric properties of the coordinating atoms in NacNac- ligands is adjustable by changes in the R substituent. Attachment to a metal center is usually carried out by initial deprotonation of HNacNac with butyllithium; the lithium derivative is then treated with a metal chloride to eliminate LiCl
. In some cases, HNacNacs also serve as charge-neutral 1,3-diimine ligands.
1,2-Diketimines, but not the 1,3-diketimines, are “non-innocent ligand
s”, akin to the dithiolene
s.
s for the polymerization
and copolymerization of ethylene and alkenes.
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
s by replacement of the carbonyl oxygen atoms with NR groups, where R = aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
, alkyl. Two families of diketimines are important in coordination chemistry and catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
: 1,2-diketimines and 1,3-diketimines.
Preparation
Diketimines are prepared by conventional condensation reactionCondensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
s that are used to convert aldehydes and ketones to imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s, Schiff base
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
s, and oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s. For example, acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
(2,4-pentanedione) and a primary alkyl- or arylamine will react, typically in acidified ethanol, to form a diketimine. 1,3-Diketimines derived from bulky amines, e.g. 2,6-disubstituted aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s, require prolonged reaction times. 1,3-Diketimines are often referred to as "HNacNac," a modification of the abbreviation Hacac used for α,β-diketones. These species can exist as a mixture of two tautomers.

Coordination complexes
The 1,2-diketimine ligands, also called α-diimines, include dimethylglyoximeDimethylglyoxime
Dimethylglyoxime is a chemical compound described by the formula CH3CCCH3. This colourless solid is the dioxime derivative of the diketone diacetyl . DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts...
as well as oxidized derivatives of o-phenylenediamine
O-Phenylenediamine
o-Phenylenediamine is a organic compound with the formula C6H42. This aromatic diamine is an important precursor to many heterocyclic compounds...
. The steric properties of the substituents on nitrogen provide a means to control the axial coordination sites on a square planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
complex. Large planar substituents such as mesityl tend to be orthogonal to the MN2 plane. In this way, the axial coordination sites on a square planar complex are shielded. Such steric control is not possible for complexes of the related to 2,2'-bipyridine
2,2'-Bipyridine
2,2'-Bipyridine is a organic compound with the formula . This colorless solid, commonly abbreviated bipy or bpy , is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals...
, glyoximate
Dimethylglyoxime
Dimethylglyoxime is a chemical compound described by the formula CH3CCCH3. This colourless solid is the dioxime derivative of the diketone diacetyl . DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts...
, and 9,10-phenanthroline
Phenanthroline
Phenanthroline is a heterocyclic organic compound. As a bidentate ligand in coordination chemistry, it forms strong complexes with most metal ions...
ligands.

Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of HNacNac compounds affords anionic bidentate ligands that form a variety of coordination complexes. Some derivatives with large R group
R group
R group may refer to:* Side chain in chemistry* Tempered representation in mathematics...
s can been used to stabilize low valent main group
P-block
The p-block of the periodic table of the elements consists of the last six groups minus helium . In the elemental form of the p-block elements, the highest energy electron occupies a p-orbital.-See also:...
and transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
complexes. Unlike the situation for the acetylacetonates, the steric properties of the coordinating atoms in NacNac- ligands is adjustable by changes in the R substituent. Attachment to a metal center is usually carried out by initial deprotonation of HNacNac with butyllithium; the lithium derivative is then treated with a metal chloride to eliminate LiCl
Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound, although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents and its hygroscopic...
. In some cases, HNacNacs also serve as charge-neutral 1,3-diimine ligands.
1,2-Diketimines, but not the 1,3-diketimines, are “non-innocent ligand
Non-innocent ligand
In chemistry, a non-innocent ligand is a ligand in a metal complex where the oxidation state is unclear. Typically, complexes containing non-innocent ligands are redox active at mild potentials...
s”, akin to the dithiolene
Dithiolene
Metal dithiolene complexes are complexes containing dithiolene ligands. Dithiolene ligands are unsaturated bidentate ligand wherein the two donor atoms are sulfur. Dithiolenes are often referred to as "metallodithiolenes" or "dithiolene complexes"...
s.
Uses
Substituted α-diimine and NacNac ligands are useful in the preparation of so-called post-metallocene catalystPost-metallocene catalyst
A post-metallocene catalyst is a kind of catalyst for olefin polymerization. "Post-metallocene" refers to the generation of catalysts following Kaminsky catalysts, which are metallocene catalysts discovered in 1980 by Walter Kaminsky, and have been highly publicized in the olefin polymerization...
s for the polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
and copolymerization of ethylene and alkenes.

