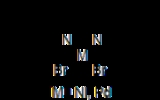
Post-metallocene catalyst
Encyclopedia
A post-metallocene catalyst is a kind of catalyst for olefin polymerization
. "Post-metallocene" refers to the generation of catalysts following Kaminsky catalyst
s, which are metallocene
catalysts discovered in 1980 by Walter Kaminsky
, and have been highly publicized in the olefin polymerization catalyst area for the past quarter century. Nonetheless, traditional Ziegler-Natta catalysts still dominate the industry.
Metallocene catalysts are homogeneous single-site systems, implying that there is a single, uniform type of catalyst present in the system. This is in contrast to the Ziegler-Natta catalysts that are heterogeneous catalysts and contain a range of catalytic sites. The catalytic properties of single-site catalysts can be controlled by modification of the structure of the catalyst. A large number of studies have been conducted by academia and industry to construct high-performance metallocene catalysts based on new concepts. One major untapped area was the copolymerization of ethylene with polar comonomers. The high oxophilicity of the early metals precluded their use in this application.
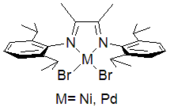 In an effort to copolymerize polar comonomers, Maurice Brookhart
In an effort to copolymerize polar comonomers, Maurice Brookhart
at the University of North Carolina
began to investigate catalysts based upon nickel
and palladium
. These catalysts were referred to as post-metallocene catalysts. They were based upon complexes bearing bulky, neutral, alpha-diimine (or diketimine
) ligands. The technology was further developed in the laboratories of DuPont
’s Central Research. They have been commercialized as DuPont’s Versipol olefin polymerization system. A significant effort at Eastman led to the related Gavilan technology that has now been incorporated into the DuPont patent estate. The catalysts are able to homopolymerize ethylene
to a variety of structures that range from high density polyethylene
through hydrocarbon plastomers and elastomers by a mechanism referred to as “chain-walking.”. By reducing the bulk of the alpha-diimine used, the product distribution of these systems can be 'tuned' to consist of hydrocarbon oils (alpha-olefins), similar to those produced by more tradition nickel(II) oligo/polymerisation catalysts. As opposed to metallocene
s, they can also randomly copolymerize ethylene with polar comonomers such as methyl acrylate
.
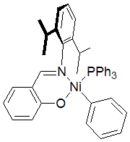 A second class of complexes were discovered simultaneously by Robert H. Grubbs
A second class of complexes were discovered simultaneously by Robert H. Grubbs
at Caltech and Lynda Johnson at DuPont. These are the complexes bearing mono-anionic bidentate ligands shown to the right. Grubbs focused primarily on salicylaldimine ligands such as the one shown, while DuPont explored them more broadly. One interesting feature of these catalysts was that pendant Lewis acid functionality could be incorporated to direct the incorporation of polar comonomers. These catalysts are often better suited for the preparation of specialty oligomers and polymers incorporating reactive functionality.
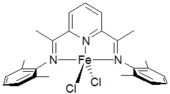 The concept of bulky bis-imine ligands was extended to iron complexes by Vernon Gibson, Maurice Brookhart
The concept of bulky bis-imine ligands was extended to iron complexes by Vernon Gibson, Maurice Brookhart
and Alison Bennett. The catalysts incorporated a pyridyl between the two imine groups giving a tridentate ligand. These catalysts, illustrated to the left, were remarkably active and no chain walking is observed in these systems. As a result, these complexes give very linear high density polyethylene when bulky and when the steric bulk is removed, they are very active catalysts for ethylene oligomerization to linear alpha-olefins.
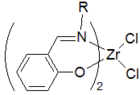 A salicylimine catalyst system based on zirconium, shown to the right, was developed by Terunori Fujita and provides extremely high activity for ethylene
A salicylimine catalyst system based on zirconium, shown to the right, was developed by Terunori Fujita and provides extremely high activity for ethylene
polymerization. The catalysts can also produce some novel polypropylene
structures, as demonstrated by Geoff Coates.
A review of the late-metal catalysts described above is available. Despite intensive research and development of these systems, very few have been successfully commercialized due to the presence of established technologies.
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
. "Post-metallocene" refers to the generation of catalysts following Kaminsky catalyst
Kaminsky catalyst
A Kaminsky catalyst is a catalytic system for alkene polymerization discovered in 1980. Kaminsky catalysts are based on metallocenes of group 4 transition metals with methylaluminoxane . These catalysts are a type of Ziegler-Natta catalyst, but they are homogeneous and show extremely high activity...
s, which are metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
catalysts discovered in 1980 by Walter Kaminsky
Walter Kaminsky
Walter Kaminsky is a German chemist. His research dwells in olefin polymerization, and also in plastic recycling. He discovered the high activity of Group 4 metallocene/methylaluminoxane mixtures as catalysts for olefin polymerization in 1980.He was awarded, among other prizes, the 1999 Benjamin...
, and have been highly publicized in the olefin polymerization catalyst area for the past quarter century. Nonetheless, traditional Ziegler-Natta catalysts still dominate the industry.
Metallocene catalysts are homogeneous single-site systems, implying that there is a single, uniform type of catalyst present in the system. This is in contrast to the Ziegler-Natta catalysts that are heterogeneous catalysts and contain a range of catalytic sites. The catalytic properties of single-site catalysts can be controlled by modification of the structure of the catalyst. A large number of studies have been conducted by academia and industry to construct high-performance metallocene catalysts based on new concepts. One major untapped area was the copolymerization of ethylene with polar comonomers. The high oxophilicity of the early metals precluded their use in this application.
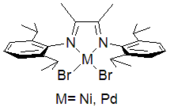
Maurice Brookhart
Maurice S. Brookhart is the William R. Kenan, Jr. Professor of Chemistry in the Department of Chemistry at the University of North Carolina....
at the University of North Carolina
University of North Carolina at Chapel Hill
The University of North Carolina at Chapel Hill is a public research university located in Chapel Hill, North Carolina, United States...
began to investigate catalysts based upon nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
and palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
. These catalysts were referred to as post-metallocene catalysts. They were based upon complexes bearing bulky, neutral, alpha-diimine (or diketimine
Diketimine
Diketimines or diimines are a family of ligands and ligand precursors derived from 1,2- and 1,3-diketones by replacement of the carbonyl oxygen atoms with NR groups, where R = aryl, alkyl...
) ligands. The technology was further developed in the laboratories of DuPont
DuPont
E. I. du Pont de Nemours and Company , commonly referred to as DuPont, is an American chemical company that was founded in July 1802 as a gunpowder mill by Eleuthère Irénée du Pont. DuPont was the world's third largest chemical company based on market capitalization and ninth based on revenue in 2009...
’s Central Research. They have been commercialized as DuPont’s Versipol olefin polymerization system. A significant effort at Eastman led to the related Gavilan technology that has now been incorporated into the DuPont patent estate. The catalysts are able to homopolymerize ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
to a variety of structures that range from high density polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
through hydrocarbon plastomers and elastomers by a mechanism referred to as “chain-walking.”. By reducing the bulk of the alpha-diimine used, the product distribution of these systems can be 'tuned' to consist of hydrocarbon oils (alpha-olefins), similar to those produced by more tradition nickel(II) oligo/polymerisation catalysts. As opposed to metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
s, they can also randomly copolymerize ethylene with polar comonomers such as methyl acrylate
Methyl acrylate
Methyl acrylate is a volatile chemical compound classified as a methyl ester. It has a characteristic acrid odor used in the preparation of polyamidoamine dendrimers typically by Michael addition with a primary amine....
.
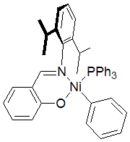
Robert H. Grubbs
Robert Howard Grubbs is an American chemist and Nobel laureate.As he noted in his official Nobel Prize autobiography, "In some places, my birthplace is listed as Calvert City and in others Possum Trot [NB: both in Marshall County]...
at Caltech and Lynda Johnson at DuPont. These are the complexes bearing mono-anionic bidentate ligands shown to the right. Grubbs focused primarily on salicylaldimine ligands such as the one shown, while DuPont explored them more broadly. One interesting feature of these catalysts was that pendant Lewis acid functionality could be incorporated to direct the incorporation of polar comonomers. These catalysts are often better suited for the preparation of specialty oligomers and polymers incorporating reactive functionality.
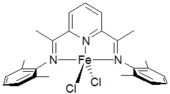
Maurice Brookhart
Maurice S. Brookhart is the William R. Kenan, Jr. Professor of Chemistry in the Department of Chemistry at the University of North Carolina....
and Alison Bennett. The catalysts incorporated a pyridyl between the two imine groups giving a tridentate ligand. These catalysts, illustrated to the left, were remarkably active and no chain walking is observed in these systems. As a result, these complexes give very linear high density polyethylene when bulky and when the steric bulk is removed, they are very active catalysts for ethylene oligomerization to linear alpha-olefins.
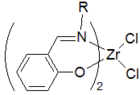
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
polymerization. The catalysts can also produce some novel polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
structures, as demonstrated by Geoff Coates.
A review of the late-metal catalysts described above is available. Despite intensive research and development of these systems, very few have been successfully commercialized due to the presence of established technologies.

