Dioxygen complex
Encyclopedia
Dioxygen complexes are coordination compounds that contain O2 as a ligand
. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin
, hemoglobin
, hemerythrin
, and hemocyanin
. Several transition metal
s form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration
, corrosion
, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2.
) or “side-on” (η2-). The bonding and structures of these compounds are usually evaluated by single-crystal X-ray crystallography
, focusing both on the overall geometry as well as the O---O distances, which reveals its bond order
.
s derived from cobalt
(II) and iron
(II) porphyrin
complexes and related anionic ligands exhibit this bonding mode. Myoglobin and hemoglobin are famous examples, and many synthetic analogues have been described that behave similarly. Binding of O2 is usually described as proceeding via electron transfer
from the metal(II) center to give superoxide
(O2-) complexes of metal(III) centers.
reversibly binds O2 (Ph = C6H5):
The conversion is described as a 2 e- redox
process: Ir(I) converts to Ir(III) as dioxygen converts to peroxide. Since O2 has a triplet ground state and Vaska's complex is a singlet, the reaction is slower than when singlet oxygen
is used.
Complexes containing η2-O2 ligands are fairly common, but most are generated using hydrogen peroxide
, not O2. Chromate
([CrO4)]2-) can for example be converted to the tetraperoxide [Cr(O2)4]2-. The reaction of hydrogen peroxide with aqueous titanium(IV) gives a brightly colored peroxy complex that is a useful test for titanium as well as hydrogen peroxide.
in fuel cell
s.
Metal-catalyzed oxidations with O2 proceed via the intermediacy of dioxygen complexes, although the actual oxidants are often oxo derivatives. The reversible binding of O2 to metal complexes has been used as a means to purify oxygen from air, but cryogenic distillation of liquid air
remains the dominant technology.
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
, hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
, hemerythrin
Hemerythrin
Hemerythrin is an oligomeric protein responsible for oxygen transport in the marine invertebrate phyla of sipunculids, priapulids, brachiopods, and in a single annelid worm, magelona. Recently, hemerythrin was discovered in methanotrophic bacterium Methylococcus capsulatus...
, and hemocyanin
Hemocyanin
Hemocyanins are respiratory proteins in the form of metalloproteins containing two copper atoms that reversibly bind a single oxygen molecule . Oxygenation causes a color change between the colorless Cu deoxygenated form and the blue Cu oxygenated form...
. Several transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
, corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2.
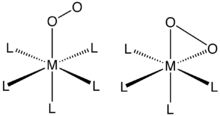
Mononuclear complexes of O2
O2 binds to a single metal center either “end-on” (η1-Hapticity
The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to...
) or “side-on” (η2-). The bonding and structures of these compounds are usually evaluated by single-crystal X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
, focusing both on the overall geometry as well as the O---O distances, which reveals its bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
.
Complexes of η1-O2 ligands
O2 adductAdduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
s derived from cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
(II) and iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
(II) porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
complexes and related anionic ligands exhibit this bonding mode. Myoglobin and hemoglobin are famous examples, and many synthetic analogues have been described that behave similarly. Binding of O2 is usually described as proceeding via electron transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
from the metal(II) center to give superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
(O2-) complexes of metal(III) centers.
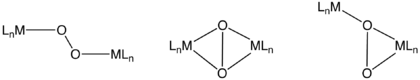
Complexes of η2-O2 ligands
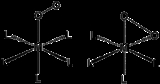
η2- bonding is the most common motif seen in coordination chemistry of dioxygen. Such complexes can generated by treating low-valent metal complexes with gaseous oxygen. For example, Vaska's complexVaska's complex
Vaska's complex is the trivial name for the chemical compound trans-chlorocarbonylbisiridium, which has the formula IrCl[P3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a...
reversibly binds O2 (Ph = C6H5):
- IrCl(CO)(PPh3)2 + O2
 IrCl(CO)(PPh3)2O2
IrCl(CO)(PPh3)2O2
The conversion is described as a 2 e- redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
process: Ir(I) converts to Ir(III) as dioxygen converts to peroxide. Since O2 has a triplet ground state and Vaska's complex is a singlet, the reaction is slower than when singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
is used.
Complexes containing η2-O2 ligands are fairly common, but most are generated using hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, not O2. Chromate
Chromate
Chromate salts contain the chromate anion, CrO42−. Dichromate salts contain the dichromate anion, Cr2O72−. They are oxyanions of chromium in the oxidation state +6. They are moderately strong oxidizing agents.- Chemical properties :...
([CrO4)]2-) can for example be converted to the tetraperoxide [Cr(O2)4]2-. The reaction of hydrogen peroxide with aqueous titanium(IV) gives a brightly colored peroxy complex that is a useful test for titanium as well as hydrogen peroxide.
Binuclear complexes of O2
O2 can bind to one metal of a bimetallic unit via the same modes discussed above for mononuclear complexes. A well known example in nature is hemerythrin, which features a diiron carboxylate that binds O2 at one Fe center. Dinuclear complexes can also cooperate in the binding, although the initial attack of O2 probably occurs at a single metal. These binding modes include μ2-η2,η2-, μ2-η1,η1-, and μ2-η1,η2-. Depending on the degree of electron-transfer from the dimetal unit, these O2 ligands can again be described as peroxo or superoxo. In nature, such dinuclear dioxygen complexes often feature copper.Relationship to other oxygenic ligands and applications
Dioxygen complexes are the precursors to other families of oxygenic ligands. Metal oxo compounds arise from the cleavage of the O-O bond after complexation. Hydroperoxo complexes are generated in the course of the reduction of dioxygen by metals. The reduction of O2 by metal catalysts is a key half-reactionHalf-reaction
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction.-Example:...
in fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s.
Metal-catalyzed oxidations with O2 proceed via the intermediacy of dioxygen complexes, although the actual oxidants are often oxo derivatives. The reversible binding of O2 to metal complexes has been used as a means to purify oxygen from air, but cryogenic distillation of liquid air
Liquid air
Liquid air is air that has been cooled to very low temperatures so that it has condensed to a pale blue mobile liquid. To protect it from room temperature, it must be kept in a vacuum flask. Liquid air can absorb heat rapidly and revert to its gaseous state...
remains the dominant technology.