
Duane–Hunt law
Encyclopedia

William Duane (physicist)
William Duane was an American physicist. A coworker of Marie Curie, he developed a method for generating quantities of radon in the laboratory.-Biography:-Studies:...
and Franklin Hunt, gives the maximum frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of X-rays that can be emitted by Bremsstrahlung
Bremsstrahlung
Bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
in an X-ray tube
X-ray tube
An X-ray tube is a vacuum tube that produces X-rays. They are used in X-ray machines. X-rays are part of the electromagnetic spectrum, an ionizing radiation with wavelengths shorter than ultraviolet light...
by accelerating electrons through an excitation voltage
Voltage
Voltage, otherwise known as electrical potential difference or electric tension is the difference in electric potential between two points — or the difference in electric potential energy per unit charge between two points...
V into a metal target.
The maximum frequency νmax is given by
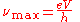
which corresponds to a minimum wavelength
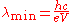
where h is Planck's constant, e is the charge of the electron, and c is the speed of light
Speed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
. This can also be written as:
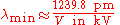 .
.The process of X-ray emission by incoming electrons is also known as the inverse photoelectric effect
Photoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
.
Explanation
In an X-ray tubeX-ray tube
An X-ray tube is a vacuum tube that produces X-rays. They are used in X-ray machines. X-rays are part of the electromagnetic spectrum, an ionizing radiation with wavelengths shorter than ultraviolet light...
, electrons are accelerated in a vacuum by an electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
and shot into a piece of metal called the "target". X-rays are emitted as the electrons slow down (decelerate) in the metal. The output spectrum consists of a continuous spectrum of X-rays, with additional sharp peaks at certain energies (see graph on right). The continuous spectrum is due to bremsstrahlung
Bremsstrahlung
Bremsstrahlung is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon because energy is conserved. The term is...
, while the sharp peaks are characteristic X-rays
Characteristic x-ray
A high energy electron interacts with a bound electron in an atom and ejects it. The incident electron is scattered and the target electron gets displaced from its shell. The incident electron energy must exceed the binding energy of the electron to eject it...
associated with the atoms in the target.
The spectrum has a sharp cutoff at low wavelength (high frequency), which is due to the limited energy of the incoming electrons. For example, if each electron in the tube is accelerated through 60 kV, then it will acquire a kinetic energy of 60 keV, and when it strikes the target it can create X-ray photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s with energy of at most 60 keV, by conservation of energy
Conservation of energy
The nineteenth century law of conservation of energy is a law of physics. It states that the total amount of energy in an isolated system remains constant over time. The total energy is said to be conserved over time...
. (This upper limit corresponds to the electron coming to a stop by emitting just one X-ray photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
. Usually the electron emits many photons, and each has an energy less than 60 keV.) A photon with energy of 60 keV or less has a wavelength of 21 pm or more, so the X-ray spectrum has exactly that cutoff, as seen in the graph. This cutoff applies to both the continuous (bremsstrahlung) spectrum and the characteristic sharp peaks
Characteristic x-ray
A high energy electron interacts with a bound electron in an atom and ejects it. The incident electron is scattered and the target electron gets displaced from its shell. The incident electron energy must exceed the binding energy of the electron to eject it...
: There is no X-ray of any kind beyond the cutoff. However, the cutoff is most obvious for the continuous spectrum.
The exact formula for the cutoff comes from setting equal the kinetic energy of the electron, , and the energy of the X-ray photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
, .

