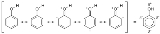
Electron density
Encyclopedia
Electron density is the measure of the probability
of an electron
being present at a specific location.
In molecule
s, regions of electron density are usually found around the atom
, and its bonds. In de-localized or conjugated system
s, such as phenol
, benzene
and compounds such as hemoglobin
and chlorophyll
, the electron density covers an entire region, i.e., in benzene they are found above and below the planar ring. This is sometimes shown diagrammatically as a series of alternating single and double bonds. In the case of phenol and benzene, a circle inside a hexagon shows the de-localized nature of the compound. This is shown below:
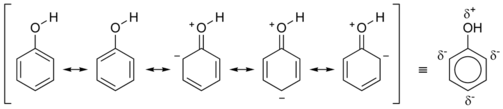 In compounds with multiple ring systems which are interconnected, this is no longer accurate, so alternating single and double bonds are used. In compounds such as chlorophyll and phenol, some diagrams show a dotted or dashed line to represent the de-localization of areas where the electron density is higher next to the single bonds. Conjugated systems can sometimes represent regions where electromagnetic radiation
In compounds with multiple ring systems which are interconnected, this is no longer accurate, so alternating single and double bonds are used. In compounds such as chlorophyll and phenol, some diagrams show a dotted or dashed line to represent the de-localization of areas where the electron density is higher next to the single bonds. Conjugated systems can sometimes represent regions where electromagnetic radiation
is absorbed at different wavelengths resulting in compounds appearing coloured. In polymer
s, these areas are known as chromophores.
In quantum chemical calculations
, the electron density, ρ(r), is a function of the coordinates r, defined so ρ(r)dr is the number of electrons in a small volume dr. For closed-shell molecules, ρ(r) can be written in terms of a sum of products of basis functions, φ:
P is the density matrix
. Electron densities are often be rendered in terms of an isosurface (an isodensity surface) with the size and shape of the surface determined by the value of the density chosen, or in terms of a percentage of total electrons enclosed.
Molecular modeling software often provides graphical images of electron density. For example, in aniline
(see image at right). Graphical models, including electron density are a commonly employed tool in chemistry education. Note in the left-most image of aniline, high electron densities are associated with the carbon
s and nitrogen
, but the hydrogen
s with only one proton in their nuclei, are not visible. This is the reason that X-ray diffraction has a difficult time locating hydrogen positions.
Most molecular modeling software packages allow the user to choose a value for the electron density, often called the IsoValue. Some software also allows for specification of the electron density in terms of percentage of total electrons enclosed. Depending on the IsoValue (typical units are electrons per cubic bohr
), or the percentage of total electrons enclosed, the electron density surface can be used locate atoms, emphasize electron densities associated with chemical bond
s, or to indicate overall molecular size and shape.
Graphically, the electron density surface also serves as a canvas upon which other electronic properties can be displayed. The electrostatic potential map (the property of electrostatic potential mapped upon the electron density) provides an indicator for charge distribution in a molecule. The local ionization potential map (the property of local ioniozation potential
mapped upon the electron density) provides an indicator of electrophilicity. And the LUMO map (lowest unoccupied molecular orbital
mapped upon the electron density) can provide an indicatory for nucleophilicity.
Electron densities are often probed with X-ray diffraction scans, where X-rays of a suitable wavelength are targeted towards a sample and measurements are made over time to represent, probabilistically, where electrons can be found, from these positions molecular structures can often be determined for crystallized systems. Quantum electrodynamics
and some branches of quantum theory
also study and analyze electron superposition
and other phenomena. Quantum tunneling and quantum entanglement
are interesting areas involving electron
s (or photon
s). High speed electrons are often used in transmission electron microscopy
(TEM) and deep inelastic scattering
, as well as many other high-speed particle experiments involving electrons.
Mulliken population analysis is based on electron densities in molecules and is a way of dividing the density between atoms to give an estimate of atomic charges.
Probability
Probability is ordinarily used to describe an attitude of mind towards some proposition of whose truth we arenot certain. The proposition of interest is usually of the form "Will a specific event occur?" The attitude of mind is of the form "How certain are we that the event will occur?" The...
of an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
being present at a specific location.
In molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, regions of electron density are usually found around the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
, and its bonds. In de-localized or conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
s, such as phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
, benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and compounds such as hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
and chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
, the electron density covers an entire region, i.e., in benzene they are found above and below the planar ring. This is sometimes shown diagrammatically as a series of alternating single and double bonds. In the case of phenol and benzene, a circle inside a hexagon shows the de-localized nature of the compound. This is shown below:

Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
is absorbed at different wavelengths resulting in compounds appearing coloured. In polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s, these areas are known as chromophores.
In quantum chemical calculations
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
, the electron density, ρ(r), is a function of the coordinates r, defined so ρ(r)dr is the number of electrons in a small volume dr. For closed-shell molecules, ρ(r) can be written in terms of a sum of products of basis functions, φ:
P is the density matrix
Density matrix
In quantum mechanics, a density matrix is a self-adjoint positive-semidefinite matrix of trace one, that describes the statistical state of a quantum system...
. Electron densities are often be rendered in terms of an isosurface (an isodensity surface) with the size and shape of the surface determined by the value of the density chosen, or in terms of a percentage of total electrons enclosed.
Molecular modeling software often provides graphical images of electron density. For example, in aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
(see image at right). Graphical models, including electron density are a commonly employed tool in chemistry education. Note in the left-most image of aniline, high electron densities are associated with the carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
s and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, but the hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
s with only one proton in their nuclei, are not visible. This is the reason that X-ray diffraction has a difficult time locating hydrogen positions.
Most molecular modeling software packages allow the user to choose a value for the electron density, often called the IsoValue. Some software also allows for specification of the electron density in terms of percentage of total electrons enclosed. Depending on the IsoValue (typical units are electrons per cubic bohr
Bohr radius
The Bohr radius is a physical constant, approximately equal to the most probable distance between the proton and electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an atom...
), or the percentage of total electrons enclosed, the electron density surface can be used locate atoms, emphasize electron densities associated with chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s, or to indicate overall molecular size and shape.
Graphically, the electron density surface also serves as a canvas upon which other electronic properties can be displayed. The electrostatic potential map (the property of electrostatic potential mapped upon the electron density) provides an indicator for charge distribution in a molecule. The local ionization potential map (the property of local ioniozation potential
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
mapped upon the electron density) provides an indicator of electrophilicity. And the LUMO map (lowest unoccupied molecular orbital
Lumo
Lumo is a 2007 documentary film about twenty-year-old Lumo Sinai, a woman who fell victim to "Africa's First World War." While returning home one day, Lumo and another woman were gang-raped by a group of soldiers fighting for control of the Democratic Republic of the Congo during the 1994 Rwandan...
mapped upon the electron density) can provide an indicatory for nucleophilicity.
Electron densities are often probed with X-ray diffraction scans, where X-rays of a suitable wavelength are targeted towards a sample and measurements are made over time to represent, probabilistically, where electrons can be found, from these positions molecular structures can often be determined for crystallized systems. Quantum electrodynamics
Quantum electrodynamics
Quantum electrodynamics is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved...
and some branches of quantum theory
Quantum field theory
Quantum field theory provides a theoretical framework for constructing quantum mechanical models of systems classically parametrized by an infinite number of dynamical degrees of freedom, that is, fields and many-body systems. It is the natural and quantitative language of particle physics and...
also study and analyze electron superposition
Superposition principle
In physics and systems theory, the superposition principle , also known as superposition property, states that, for all linear systems, the net response at a given place and time caused by two or more stimuli is the sum of the responses which would have been caused by each stimulus individually...
and other phenomena. Quantum tunneling and quantum entanglement
Quantum entanglement
Quantum entanglement occurs when electrons, molecules even as large as "buckyballs", photons, etc., interact physically and then become separated; the type of interaction is such that each resulting member of a pair is properly described by the same quantum mechanical description , which is...
are interesting areas involving electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s (or photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s). High speed electrons are often used in transmission electron microscopy
Transmission electron microscopy
Transmission electron microscopy is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen, interacting with the specimen as it passes through...
(TEM) and deep inelastic scattering
Inelastic scattering
In particle physics and chemistry, inelastic scattering is a fundamental scattering process in which the kinetic energy of an incident particle is not conserved . In an inelastic scattering process, some of the energy of the incident particle is lost or gained...
, as well as many other high-speed particle experiments involving electrons.
Mulliken population analysis is based on electron densities in molecules and is a way of dividing the density between atoms to give an estimate of atomic charges.
Spin density
Spin density is electron density applied to free radicals. It is defined as the total electron density of electrons of one spin minus the total electron density of the electrons of the other spin. One of the ways to measure it experimentally is by electron spin resonance, neutron diffraction allows direct mapping of the spin density in 3D-space.See also
- Difference density mapDifference density mapIn protein crystallography, a difference density map shows the spatial distribution of the difference between the measured electron density of the crystal and the electron density explained by the current model....
- Electron cloud
- Electron configurationElectron configurationIn atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
- Resolution (electron density)Resolution (electron density)Resolution in terms of electron density is a measure of the resolvability in the electron density map of a molecule. In X-ray crystallography, resolution is the highest resolvable peak in the diffraction pattern...
- Charge densityCharge densityThe linear, surface, or volume charge density is the amount of electric charge in a line, surface, or volume, respectively. It is measured in coulombs per meter , square meter , or cubic meter , respectively, and represented by the lowercase Greek letter Rho . Since there are positive as well as...

