
Bohr radius
Encyclopedia
The Bohr radius is a physical constant
, approximately equal to the most probable distance between the proton
and electron
in a hydrogen atom
in its ground state
. It is named after Niels Bohr
, due to its role in the Bohr model
of an atom. The precise definition of the Bohr radius is: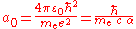
where: is the permittivity of free space
is the permittivity of free space is the reduced Planck's constant
is the reduced Planck's constant is the electron rest mass
is the electron rest mass is the elementary charge
is the elementary charge
 is the speed of light
is the speed of light
in vacuum is the fine structure constant.
is the fine structure constant.
According to 2010 CODATA the Bohr radius has a value of 5.2917721092(17) m (i.e., approximately 53 pm
or 0.53 angstrom
s).
In the Bohr model
of the structure of an atom
, put forward by Niels Bohr
in 1913, electrons orbit a central nucleus
. The model says that the electrons orbit only at certain distances from the nucleus, depending on their energy. In the simplest atom, hydrogen
, a single electron orbits the nucleus and its smallest possible orbit, with lowest energy, has an orbital radius almost equal to the Bohr radius. (It is not exactly the Bohr radius due to the reduced mass effect
. They differ by about 0.1%.)
Although the Bohr model is no longer in use, the Bohr radius remains very useful in atomic physics
calculations, due in part to its simple relationship with other fundamental constants. (This is why it is defined using the true electron mass rather than the reduced mass, as mentioned above.) For example, it is the unit of length in atomic units
.
According to the modern, quantum-mechanical
understanding of the hydrogen atom, the average distance between electron and proton is ≈1.5a0, somewhat different than the value in the Bohr model (≈a0), but certainly the same order of magnitude
.
The Bohr radius of the electron is one of a trio of related units of length, the other two being the Compton wavelength
of the electron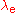 and the classical electron radius
and the classical electron radius
 . The Bohr radius is built from the electron mass
. The Bohr radius is built from the electron mass  , Planck's constant
, Planck's constant  and the electron charge
and the electron charge  . The Compton wavelength is built from
. The Compton wavelength is built from  ,
,  and the speed of light
and the speed of light  . The classical electron radius is built from
. The classical electron radius is built from  ,
,  and
and  . Any one of these three lengths can be written in terms of any other using the fine structure constant
. Any one of these three lengths can be written in terms of any other using the fine structure constant  :
:
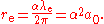
The Compton wavelength is about 20 times smaller than the Bohr radius, and the classical electron radius is about 1000 times smaller than the Compton wavelength.
in the hydrogen atom can be given by the following equation:
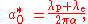
where
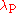 is the Compton wavelength of the proton
is the Compton wavelength of the proton
.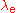 is the Compton wavelength of the electron.
is the Compton wavelength of the electron. is the fine structure constant.
is the fine structure constant.
In the above equation, the effect of the reduced mass
is achieved by using the increased Compton wavelength, which is just the Compton wavelengths of the electron and the proton added together.
Physical constant
A physical constant is a physical quantity that is generally believed to be both universal in nature and constant in time. It can be contrasted with a mathematical constant, which is a fixed numerical value but does not directly involve any physical measurement.There are many physical constants in...
, approximately equal to the most probable distance between the proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
and electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
in a hydrogen atom
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
in its ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
. It is named after Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
, due to its role in the Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of an atom. The precise definition of the Bohr radius is:
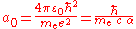
where:
 is the permittivity of free space
is the permittivity of free space is the reduced Planck's constant
is the reduced Planck's constant is the electron rest mass
is the electron rest mass is the elementary charge
is the elementary chargeElementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
 is the speed of light
is the speed of lightSpeed of light
The speed of light in vacuum, usually denoted by c, is a physical constant important in many areas of physics. Its value is 299,792,458 metres per second, a figure that is exact since the length of the metre is defined from this constant and the international standard for time...
in vacuum
 is the fine structure constant.
is the fine structure constant.According to 2010 CODATA the Bohr radius has a value of 5.2917721092(17) m (i.e., approximately 53 pm
1 E-12 m
To help compare different orders of magnitude this page lists lengths between 10−12 m and 10−11 m .Distances shorter than 1 pm* 1 pm = 1 picometre = 1,000 femtometres* 1 pm = distance between atomic nuclei in a white dwarf...
or 0.53 angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
s).
In the Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the structure of an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
, put forward by Niels Bohr
Niels Bohr
Niels Henrik David Bohr was a Danish physicist who made foundational contributions to understanding atomic structure and quantum mechanics, for which he received the Nobel Prize in Physics in 1922. Bohr mentored and collaborated with many of the top physicists of the century at his institute in...
in 1913, electrons orbit a central nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. The model says that the electrons orbit only at certain distances from the nucleus, depending on their energy. In the simplest atom, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, a single electron orbits the nucleus and its smallest possible orbit, with lowest energy, has an orbital radius almost equal to the Bohr radius. (It is not exactly the Bohr radius due to the reduced mass effect
Reduced mass
Reduced mass is the "effective" inertial mass appearing in the two-body problem of Newtonian mechanics. This is a quantity with the unit of mass, which allows the two-body problem to be solved as if it were a one-body problem. Note however that the mass determining the gravitational force is not...
. They differ by about 0.1%.)
Although the Bohr model is no longer in use, the Bohr radius remains very useful in atomic physics
Atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and...
calculations, due in part to its simple relationship with other fundamental constants. (This is why it is defined using the true electron mass rather than the reduced mass, as mentioned above.) For example, it is the unit of length in atomic units
Atomic units
Atomic units form a system of natural units which is especially convenient for atomic physics calculations. There are two different kinds of atomic units, which one might name Hartree atomic units and Rydberg atomic units, which differ in the choice of the unit of mass and charge. This article...
.
According to the modern, quantum-mechanical
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
understanding of the hydrogen atom, the average distance between electron and proton is ≈1.5a0, somewhat different than the value in the Bohr model (≈a0), but certainly the same order of magnitude
Order of magnitude
An order of magnitude is the class of scale or magnitude of any amount, where each class contains values of a fixed ratio to the class preceding it. In its most common usage, the amount being scaled is 10 and the scale is the exponent being applied to this amount...
.
The Bohr radius of the electron is one of a trio of related units of length, the other two being the Compton wavelength
Compton wavelength
The Compton wavelength is a quantum mechanical property of a particle. It was introduced by Arthur Compton in his explanation of the scattering of photons by electrons...
of the electron
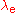 and the classical electron radius
and the classical electron radiusClassical electron radius
The classical electron radius, also known as the Lorentz radius or the Thomson scattering length, is based on a classical relativistic model of the electron...
 . The Bohr radius is built from the electron mass
. The Bohr radius is built from the electron mass  , Planck's constant
, Planck's constant  and the electron charge
and the electron charge  . The Compton wavelength is built from
. The Compton wavelength is built from  ,
,  and the speed of light
and the speed of light  . The classical electron radius is built from
. The classical electron radius is built from  ,
,  and
and  . Any one of these three lengths can be written in terms of any other using the fine structure constant
. Any one of these three lengths can be written in terms of any other using the fine structure constant  :
: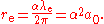
The Compton wavelength is about 20 times smaller than the Bohr radius, and the classical electron radius is about 1000 times smaller than the Compton wavelength.
Reduced Bohr radius
The Bohr radius including the effect of reduced massReduced mass
Reduced mass is the "effective" inertial mass appearing in the two-body problem of Newtonian mechanics. This is a quantity with the unit of mass, which allows the two-body problem to be solved as if it were a one-body problem. Note however that the mass determining the gravitational force is not...
in the hydrogen atom can be given by the following equation:
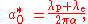
where
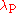 is the Compton wavelength of the proton
is the Compton wavelength of the protonProton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
.
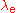 is the Compton wavelength of the electron.
is the Compton wavelength of the electron. is the fine structure constant.
is the fine structure constant.In the above equation, the effect of the reduced mass
Reduced mass
Reduced mass is the "effective" inertial mass appearing in the two-body problem of Newtonian mechanics. This is a quantity with the unit of mass, which allows the two-body problem to be solved as if it were a one-body problem. Note however that the mass determining the gravitational force is not...
is achieved by using the increased Compton wavelength, which is just the Compton wavelengths of the electron and the proton added together.

