Endohedral hydrogen fullerene
Encyclopedia
Endohedral hydrogen fullerene or H2@C60 is an endohedral fullerene
containing molecular hydrogen
. This chemical compound
has a potential application in molecular electronics
and was synthesized in 2005 at Kyoto University
by the group of Koichi Komatsu. Ordinarily the payload of endohedral fullerenes are inserted at the time of the synthesis of the fullerene itself or is introduced to the fullerene at very low yields at high temperatures and high pressure. This particular fullerene was synthesised in an unusual way in three steps starting from pristine C60 fullerene: cracking open the carbon framework, insert hydrogen gas and zipping up by organic synthesis
methods.
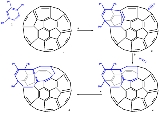
2 is fitted with two phenyl groups and a pyridine
group for reasons of solubility
and reacted in 1,2-dichlorobenzene
with pristine C60 fullerene 2 in a Diels-Alder reaction at high temperature and for an extended reaction time. In this reaction nitrogen is expulsed and an 8-membered ring is formed (3). This orifice is further extended by reaction with singlet oxygen
in carbon tetrachloride
which causes one of the ring alkene
groups to oxidize to a ketone
. The 12-ring is extended to a 13-ring by reaction with elemental sulfur
in presence of tetrakis(dimethylamino)ethylene.
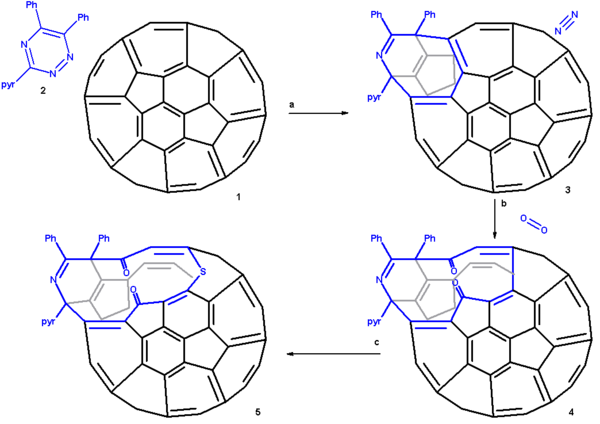 The proposed reaction mechanism
The proposed reaction mechanism
is depicted in a plat surface rendition in scheme 2. In the first step the triazine reacts with the fullerene in a Diels-Alder reaction. In the second step nitrogen is expulsed from the DA adduct 2 resulting in the formation of a fused aza-cyclohexadiene ring followed by a [4+4]cycloaddition
to an intermediate 4 with two cyclopropane
rings. This intermediate quickly rearranges in a retro [2+2+2]cycloaddition to the 8 membered ring product 5. In silico
calculations show that the electrons in the HOMO
reside primarily in the double bonds of the butadiene part of the ring and indeed singlet oxygen
reacts at these positions through the dioxetane
intermediate 6 with alkene cleavage to diketone 7 (only one isomer shown). Elemental sulfur
S8 is inserted into the single bond of the diene group leading to the extension of the ring to 13 atoms (structures 8 and 9 are identical). Tetrakis(dimethylamino)ethylene activates this bond for electrophilic sulfur addition either by one-electron reduction
or by complexation.
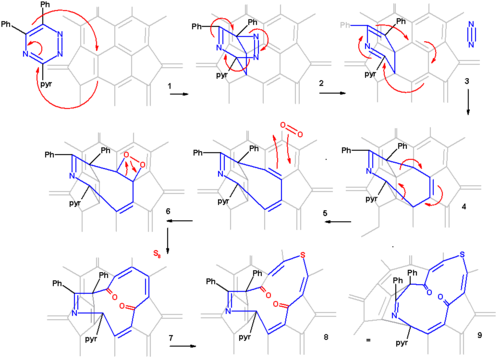 From X-ray crystallography
From X-ray crystallography
it is determined that the shape of the orifice in the sulfur compound is roughly a circle. Inserting hydrogen in this compound is an easy step taking place with 100% efficiency. Zipping up the orifice is a reversal of the steps required to open the cage. Care must be taken to keep the reaction conditions below 160 °C on order to prevent hydrogen from escaping. m-CPBA oxidizes the sulfur group to a sulfoxide
group which can then be extracted from the ring by a photochemical reaction under visible light in toluene. The two ketone groups are re-coupled in a McMurry reaction
with titanium tetrachloride
and elemental zinc
. The reverse cycloadditions take place at 340 °C in a vacuum splitting of 2-cyanopyridine and diphenylacetylene resulting in the formation of H2@C60 at a 40% chemical yield starting from pristine fullerene.
The process of hydrogen introduction and release can be facilitated by increasing the size of the orifice. This can be done by replacing sulfur by selenium
(sodium thiolate, Se8) exploiting larger C-Se bond length
. Filling cracked-open fullerene now takes 8 hours at 190 °C at 760 atmospheres
(77 MPa) of hydrogen and release between 150 °C and 180 °C is three times as fast compared to the sulfur analogue. The activation energy
for release is lowered by 0.7 kcal/mol to 28.2 kcal
/mol
(2.9 to 118 kJ/mol).
There is evidence that hydrogen in the fullerene cage is not completely shielded from the outside world as one study found that H2@C60 is more efficient at quenching
singlet oxygen
than empty C60.
Endohedral fullerenes
Endohedral fullerenes are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex was synthesized in 1985 called La@C60. The @ sign in the name reflects the notion of a small molecule trapped inside a shell...
containing molecular hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. This chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
has a potential application in molecular electronics
Molecular electronics
Molecular electronics, sometimes called moletronics, involves the study and application of molecular building blocks for the fabrication of electronic components...
and was synthesized in 2005 at Kyoto University
Kyoto University
, or is a national university located in Kyoto, Japan. It is the second oldest Japanese university, and formerly one of Japan's Imperial Universities.- History :...
by the group of Koichi Komatsu. Ordinarily the payload of endohedral fullerenes are inserted at the time of the synthesis of the fullerene itself or is introduced to the fullerene at very low yields at high temperatures and high pressure. This particular fullerene was synthesised in an unusual way in three steps starting from pristine C60 fullerene: cracking open the carbon framework, insert hydrogen gas and zipping up by organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
methods.
Organic synthesis
Scheme 1 presents an overview of the first step, the creation of a 13 membered ring orifice on the fullerene surface. A 1,2,4-triazineTriazine
A triazine is one of three organic chemicals, isomeric with each other, whose molecular formula is 333 and whose empirical formula is CHN.- Structure :...
2 is fitted with two phenyl groups and a pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
group for reasons of solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
and reacted in 1,2-dichlorobenzene
1,2-Dichlorobenzene
1,2-Dichlorobenzene, or orthodichlorobenzene , is an organic compound with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents...
with pristine C60 fullerene 2 in a Diels-Alder reaction at high temperature and for an extended reaction time. In this reaction nitrogen is expulsed and an 8-membered ring is formed (3). This orifice is further extended by reaction with singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
in carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
which causes one of the ring alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
groups to oxidize to a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. The 12-ring is extended to a 13-ring by reaction with elemental sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
in presence of tetrakis(dimethylamino)ethylene.

Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is depicted in a plat surface rendition in scheme 2. In the first step the triazine reacts with the fullerene in a Diels-Alder reaction. In the second step nitrogen is expulsed from the DA adduct 2 resulting in the formation of a fused aza-cyclohexadiene ring followed by a [4+4]cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
to an intermediate 4 with two cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
rings. This intermediate quickly rearranges in a retro [2+2+2]cycloaddition to the 8 membered ring product 5. In silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
calculations show that the electrons in the HOMO
Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
reside primarily in the double bonds of the butadiene part of the ring and indeed singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
reacts at these positions through the dioxetane
Oxetane
Oxetane, or 1,3-propylene oxide, is an heterocyclic organic compound with the molecular formula C3H6O, having a four-membered ring with three carbon atoms and one oxygen atom....
intermediate 6 with alkene cleavage to diketone 7 (only one isomer shown). Elemental sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
S8 is inserted into the single bond of the diene group leading to the extension of the ring to 13 atoms (structures 8 and 9 are identical). Tetrakis(dimethylamino)ethylene activates this bond for electrophilic sulfur addition either by one-electron reduction
One-electron reduction
A one-electron reduction in organic chemistry involves the transfer of an electron from a metal to an organic substrate. It serves to differentiate between true organic reductions and other reductions such as hydride transfer reactions that actually involve two-electron species.The first...
or by complexation.

X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
it is determined that the shape of the orifice in the sulfur compound is roughly a circle. Inserting hydrogen in this compound is an easy step taking place with 100% efficiency. Zipping up the orifice is a reversal of the steps required to open the cage. Care must be taken to keep the reaction conditions below 160 °C on order to prevent hydrogen from escaping. m-CPBA oxidizes the sulfur group to a sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
group which can then be extracted from the ring by a photochemical reaction under visible light in toluene. The two ketone groups are re-coupled in a McMurry reaction
McMurry reaction
The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to an alkene using titanium chloride compound such as titanium chloride and a reducing agent. The reaction is named after its co-discoverer, John E. McMurry. The McMurry reaction originally involved the...
with titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
and elemental zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
. The reverse cycloadditions take place at 340 °C in a vacuum splitting of 2-cyanopyridine and diphenylacetylene resulting in the formation of H2@C60 at a 40% chemical yield starting from pristine fullerene.
Properties
H2@C60 is found to be a stable molecule. it survives 10 minutes at 500 °C and shows the same chemical reactivity as empty C60. The electronic properties are also largely unaffected.The process of hydrogen introduction and release can be facilitated by increasing the size of the orifice. This can be done by replacing sulfur by selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
(sodium thiolate, Se8) exploiting larger C-Se bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
. Filling cracked-open fullerene now takes 8 hours at 190 °C at 760 atmospheres
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
(77 MPa) of hydrogen and release between 150 °C and 180 °C is three times as fast compared to the sulfur analogue. The activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
for release is lowered by 0.7 kcal/mol to 28.2 kcal
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
(2.9 to 118 kJ/mol).
There is evidence that hydrogen in the fullerene cage is not completely shielded from the outside world as one study found that H2@C60 is more efficient at quenching
Quenching (fluorescence)
Quenching refers to any process which decreases the fluorescence intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisional quenching. As a consequence, quenching is often heavily dependent on...
singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
than empty C60.

