
Fenske equation
Encyclopedia
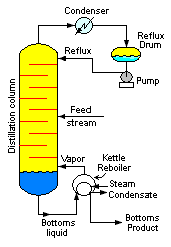
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
is an equation
Equation
An equation is a mathematical statement that asserts the equality of two expressions. In modern notation, this is written by placing the expressions on either side of an equals sign , for examplex + 3 = 5\,asserts that x+3 is equal to 5...
used for calculating the minimum number of theoretical plate
Theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
s required for the separation of a binary feed stream by a fractionation column
Fractionating column
A fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities...
that is being operated at total reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
(i.e., which means that no overhead product distillate is being withdrawn from the column).
The equation was derived by Merrell Fenske in 1932 , a professor who served as the head of the chemical engineering
Chemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
department at the Pennsylvania State University
Pennsylvania State University
The Pennsylvania State University, commonly referred to as Penn State or PSU, is a public research university with campuses and facilities throughout the state of Pennsylvania, United States. Founded in 1855, the university has a threefold mission of teaching, research, and public service...
from 1959 to 1969.
This is one of the many different but equivalent versions of the Fenske equation:

| where: | |
 |
= minimum number of theoretical plates required at total reflux (of which the reboiler is one) |
|---|---|
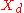 |
= mole fraction of more volatile Volatility (chemistry) In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily... component in the overhead distillate |
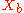 |
= mole fraction of more volatile component in the bottoms |
 |
= average relative volatility Relative volatility Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of using distillation to separate the more volatile... of more volatile component to less volatile component |
For ease of expression, the more volatile and the less volatile components are commonly referred to as the light key (LK) and the heavy key (HK), respectively.
If the relative volatility
Relative volatility
Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of using distillation to separate the more volatile...
of the light key to the heavy key is constant from the column top to the column bottom, then
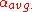 is simply
is simply  . If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used:
. If the relative volatility is not constant from top to bottom of the column, then the following approximation may be used: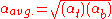
| where: | |
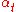 |
= relative volatility of light key to heavy key at top of column |
|---|---|
 |
= relative volatility of light key to heavy key at bottom of column |
Two comments should be made regarding the above discussion. First, in multi-component distillation, there may be a wide gap between the volatilities of the "lights" and the "heavies." In this case, all the lights can be grouped into one pseudo-component, with their properties being averaged on a mole-fraction basis, and used as the light key. Likewise, all the heavies can be grouped into another pseudo-component and used as the heavy key. Second, in columns in which the bubble point
Bubble point
When heating a liquid consisting of two or more components, the bubble point is the point where first bubble of vapor is formed. Given that vapor will probably have a different composition than the liquid, the bubble point at different compositions are useful data when designing distillation...
at the top of the column is much lower than the bubble point at the bottom, the assumption that the relative volatility of the heavy and light keys is constant over the length of the column is not appropriate, and hence, averaging the two values of alpha is not a reasonable approach. This is the case, for example, in so-called "stabilizer" columns used to purge residual lights after a flash drum. In such cases, however, the wide difference in volatilities means that separation is in fact quite easy, few stages are required, and hence the final design of the column is fairly insensitive, from the perspective of cost, to error in the value of alpha used. Hence, the smaller value (i.e. the value at the bottom of the column) can safely be used.
The above Fenske equation can be modified for use in the total reflux distillation of multi-component feeds. The Fenske Equation is also helpful in solving liquid-liquid extraction
Liquid-liquid extraction
Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid phase into another liquid...
problems, because an extraction system can also be represented as a series of equilibrium stages, and rather than relative volatility, relative solubility can be substituted.
Another form of the Fenske equation
A derivation of another form of the Fenske equation for use in gas chromatography is available on the U.S. Naval Academy's web site. Using Raoult's law and Dalton's LawDalton's law
In chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
for a series of condensation and evaporation cycles (i.e., equilibrium stages), the following form of the Fenske equation is obtained:

| where: | |
 |
= number of equilibrium stages |
|---|---|
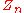 |
= mole fraction of component n in the vapor phase |
 |
= mole fraction of component n in the liquid phase |
 |
= vapor pressure Vapor pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form... of pure component n |
See also
- Continuous distillationContinuous distillationContinuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. A distillation is the separation or partial separation of a liquid feed mixture into components or...
- DistillationDistillationDistillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
- Fractional distillationFractional distillationFractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
- Fractionating columnFractionating columnA fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities...
- McCabe-Thiele methodMcCabe-Thiele methodThe McCabe-Thiele method was presented by two graduate students at Massachusetts Institute of Technology , Warren L. McCabe and Ernest W. Thiele in 1925. The technique is considered to be the simplest and perhaps most instructive method for analysis of binary distillation...
External links
- Lecture Notes (R.M. Price, Christian Brothers UniversityChristian Brothers UniversityChristian Brothers University is the oldest collegiate degree-granting institution in the city of Memphis. The university is run by the Christian Brothers, a Roman Catholic religious order founded by St. John Baptist de la Salle, the patron saint of teachers...
, Tennessee) - Studies in Chemical Process Design and Synthesis, Y. A. Liu, T.E. Quantrille, and S. Chengt, Ind. Eng. Chem. Res., Volume 29, 1990
- Multi-component Distillation (M.B. Jennings, San Jose State UniversitySan José State UniversitySan Jose State University is a public university located in San Jose, California, United States...
)

