Fertile soil
Encyclopedia
Fertile soil has the following properties:
In lands used for agriculture and other human activities, fertile soil typically arises from the use of soil conservation practices.
nitrogen is the element in soil that is most often lacking. Phosphorus and potassium are also needed in substantial amounts. For this reason these three elements are always included in commercial fertilizers, and the content of each of these items is included on the bags of fertilizer. For example a 10-10-15 fertilizer has 10 percent nitrogen, 10 percent (P2O5) available phosphorus and 15 percent (K2O) water soluble potassium.
Inorganic fertilizers are generally less expensive and have higher concentrations of nutrients than organic fertilizers. Some have criticized the use of inorganic fertilizers, claiming that the water-soluble nitrogen doesn't provide for the long-term needs of the plant and creates water pollution. Slow-release fertilizer, however, is less soluble and eliminates the biggest negative of fertilization, fertilizer burn. Additionally, most soluble fertilizers are coated, such as sulfur-coated urea.
In 2008 the cost of phosphorus as fertilizer more than doubled, while the price of rock phosphate as base commodity rose eight-fold. Recently the term peak phosphorus
has been coined, due to the limited occurrence of rock phosphate http://www.apda.pt/apda_resources/APDA.Biblioteca/eureau%5Cposition%20papers%5Cthe%20reuse%20of%20phosphorus.pdf in the world.
Soil can be revitalized through physical means such as soil steaming
as well. Superheated steam is induced into the soil to kill pests and unblock nutrients.
This is the process whereby plants use light energy to drive chemical reactions which convert CO2 into sugars. As such, all plants require access to both light and carbon dioxide to produce energy, grow and reproduce.
While typically limited by nitrogen, phosphorus and potassium, low levels of carbon dioxide can also act as a limiting factor on plant growth. Peer-reviewed and published scientific studies have shown that increasing CO2 is highly effective at promoting plant growth up to levels over 300 ppm. Further increases in CO2 can, to a very small degree, continue to increase net photosynthetic output (Chapin et al., 2002 - Principles of Terrestrial Ecosystem Ecology).
Since higher levels of CO2 have only a minimal impact on photosynthetic output at present levels (presently around 380 ppm and increasing), we should not consider plant growth to be limited by carbon dioxide. Other biochemical limitations, such as soil organic content, nitrogen in the soil, phosphorus and potassium, are far more often in short supply. As such, neither commercial nor scientific communities look to air fertilization as an effective or economic method of increasing production in agriculture or natural ecosystems. Furthermore, since microbial decomposition occurs faster under warmer temperatures, higher levels of CO2 (which is one of the causes of unusually fast climate change) should be expected to increase the rate at which nutrients are leached out of soils and may have a negative impact on soil fertility.
.
One of the most widespread occurrences of soil depletion is in tropical zones where nutrient content of soils is low. The combined effects of growing population densities, large-scale industrial logging, slash-and-burn agriculture and ranching, and other factors, have in some places depleted soils through rapid and almost total nutrient removal.
Topsoil depletion occurs when the nutrient-rich organic topsoil, which takes hundreds to thousands of years to build up under natural conditions, is eroded or depleted of its original organic material. Historically, many past civilizations' collapses can be attributed to the depletion of the topsoil. Since the beginning of agricultural production in the Great Plains
of North America in the 1880s, about one-half of its topsoil has disappeared.
Depletion may occur through a variety of other effects, including overtillage (which damages soil structure), overuse of inputs such as synthetic fertilizers and herbicides (which leave residues and buildups that inhibit microorganisms), and salinization of soil.
- It is rich in nutrients necessary for basic plant nutrition, including nitrogenNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, phosphorusPhosphorusPhosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
and potassiumPotassiumPotassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
. - It contains sufficient minerals (trace elements) for plant nutrition, including boronBoronBoron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
, chlorineChlorineChlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, cobaltCobaltCobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
, copperCopperCopper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, manganeseManganeseManganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
, magnesiumMagnesiumMagnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
, molybdenumMolybdenumMolybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
, sulfurSulfurSulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
, and zincZincZinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
. - It contains soil organic matterSoil organic matterOrganic matter is matter that has come from a once-living organism; is capable of decay, or the product of decay; or is composed of organic compounds...
that improves soil structureSoil structureSoil structure is determined by how individual soil granules clump or bind together and aggregate, and therefore, the arrangement of soil pores between them...
and soil moisture retention. - Soil pHSoil pHThe soil pH is a measure of the acidity or basicity in soils. pH is defined as the negative logarithm of the activity of hydrogen ions in solution. It ranges from 0 to 14, with 7 being neutral. A pH below 7 is acidic and above 7 is basic. Soil pH is considered a master variable in soils as it...
is in the range 6.0 to 6.8 for most plants but some prefer acid or alkaline conditions. - Good soil structure, creating well drainedDrainageDrainage is the natural or artificial removal of surface and sub-surface water from an area. Many agricultural soils need drainage to improve production or to manage water supplies.-Early history:...
soil, but some soils are wetter (as for producing riceRiceRice is the seed of the monocot plants Oryza sativa or Oryza glaberrima . As a cereal grain, it is the most important staple food for a large part of the world's human population, especially in East Asia, Southeast Asia, South Asia, the Middle East, and the West Indies...
) or drier (as for producing plants susceptible to fungi or rot) such as agaveAgaveAgave is a genus of monocots. The plants are perennial, but each rosette flowers once and then dies ; they are commonly known as the century plant....
. - A range of microorganismsSoil lifeSoil life or soil biota is a collective term for all the organisms living within the soil.-Overview:In balanced soil, plants grow in an active and steady environment. The mineral content of the soil and its heartiful structure are important for their well-being, but it is the life in the earth that...
that support plant growth. - It often contains large amounts of topsoilTopsoilTopsoil is the upper, outermost layer of soil, usually the top to . It has the highest concentration of organic matter and microorganisms and is where most of the Earth's biological soil activity occurs.-Importance:...
.
In lands used for agriculture and other human activities, fertile soil typically arises from the use of soil conservation practices.
Soil Fertilization
BioavailableBioavailability
In pharmacology, bioavailability is a subcategory of absorption and is used to describe the fraction of an administered dose of unchanged drug that reaches the systemic circulation, one of the principal pharmacokinetic properties of drugs. By definition, when a medication is administered...
nitrogen is the element in soil that is most often lacking. Phosphorus and potassium are also needed in substantial amounts. For this reason these three elements are always included in commercial fertilizers, and the content of each of these items is included on the bags of fertilizer. For example a 10-10-15 fertilizer has 10 percent nitrogen, 10 percent (P2O5) available phosphorus and 15 percent (K2O) water soluble potassium.
Inorganic fertilizers are generally less expensive and have higher concentrations of nutrients than organic fertilizers. Some have criticized the use of inorganic fertilizers, claiming that the water-soluble nitrogen doesn't provide for the long-term needs of the plant and creates water pollution. Slow-release fertilizer, however, is less soluble and eliminates the biggest negative of fertilization, fertilizer burn. Additionally, most soluble fertilizers are coated, such as sulfur-coated urea.
In 2008 the cost of phosphorus as fertilizer more than doubled, while the price of rock phosphate as base commodity rose eight-fold. Recently the term peak phosphorus
Hubbert peak theory
The Hubbert peak theory posits that for any given geographical area, from an individual oil-producing region to the planet as a whole, the rate of petroleum production tends to follow a bell-shaped curve...
has been coined, due to the limited occurrence of rock phosphate http://www.apda.pt/apda_resources/APDA.Biblioteca/eureau%5Cposition%20papers%5Cthe%20reuse%20of%20phosphorus.pdf in the world.
Soil can be revitalized through physical means such as soil steaming
Soil steam sterilization
Soil steam sterilization is a farming technique that sterilizes soil with steam in open fields or greenhouses. Pests of plant cultures such as weeds, bacteria, fungi and viruses are killed through induced hot steam which causes their cell structure to physically degenerate. Biologically, the...
as well. Superheated steam is induced into the soil to kill pests and unblock nutrients.
Light and CO2 limitations
PhotosynthesisPhotosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
This is the process whereby plants use light energy to drive chemical reactions which convert CO2 into sugars. As such, all plants require access to both light and carbon dioxide to produce energy, grow and reproduce.
While typically limited by nitrogen, phosphorus and potassium, low levels of carbon dioxide can also act as a limiting factor on plant growth. Peer-reviewed and published scientific studies have shown that increasing CO2 is highly effective at promoting plant growth up to levels over 300 ppm. Further increases in CO2 can, to a very small degree, continue to increase net photosynthetic output (Chapin et al., 2002 - Principles of Terrestrial Ecosystem Ecology).
Since higher levels of CO2 have only a minimal impact on photosynthetic output at present levels (presently around 380 ppm and increasing), we should not consider plant growth to be limited by carbon dioxide. Other biochemical limitations, such as soil organic content, nitrogen in the soil, phosphorus and potassium, are far more often in short supply. As such, neither commercial nor scientific communities look to air fertilization as an effective or economic method of increasing production in agriculture or natural ecosystems. Furthermore, since microbial decomposition occurs faster under warmer temperatures, higher levels of CO2 (which is one of the causes of unusually fast climate change) should be expected to increase the rate at which nutrients are leached out of soils and may have a negative impact on soil fertility.
Soil depletion
Soil depletion occurs when the components which contribute to fertility are removed and not replaced, and the conditions which support soil fertility are not maintained. This leads to poor crop yields. In agriculture, depletion can be due to excessively intense cultivation and inadequate soil managementSoil management
Soil management concerns all operations, practices and treatments used to protect soil and enhance its performance.-Practices:Soil management practices that affect soil quality:...
.
One of the most widespread occurrences of soil depletion is in tropical zones where nutrient content of soils is low. The combined effects of growing population densities, large-scale industrial logging, slash-and-burn agriculture and ranching, and other factors, have in some places depleted soils through rapid and almost total nutrient removal.
Topsoil depletion occurs when the nutrient-rich organic topsoil, which takes hundreds to thousands of years to build up under natural conditions, is eroded or depleted of its original organic material. Historically, many past civilizations' collapses can be attributed to the depletion of the topsoil. Since the beginning of agricultural production in the Great Plains
Great Plains
The Great Plains are a broad expanse of flat land, much of it covered in prairie, steppe and grassland, which lies west of the Mississippi River and east of the Rocky Mountains in the United States and Canada. This area covers parts of the U.S...
of North America in the 1880s, about one-half of its topsoil has disappeared.
Depletion may occur through a variety of other effects, including overtillage (which damages soil structure), overuse of inputs such as synthetic fertilizers and herbicides (which leave residues and buildups that inhibit microorganisms), and salinization of soil.
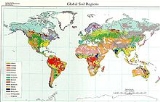
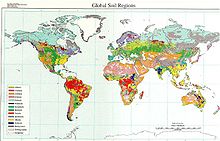
Global Distribution

See also
- Plaggen soilPlaggen soilPlaggen is a type of soil created in Europe in the Middle Ages, as a result of so called 'plaggen cultivation', created by cutting turves of peat from an outfield area, and then using them as bedding for cattle; the slurry-soaked bedding was later spread on the arable fields as fertilizer...
- Shifting cultivationShifting cultivationShifting cultivation is an agricultural system in which plots of land are cultivated temporarily, then abandoned. This system often involves clearing of a piece of land followed by several years of wood harvesting or farming, until the soil loses fertility...
- Soil contaminationSoil contaminationSoil contamination or soil pollution is caused by the presence of xenobiotic chemicals or other alteration in the natural soil environment....
- Soil lifeSoil lifeSoil life or soil biota is a collective term for all the organisms living within the soil.-Overview:In balanced soil, plants grow in an active and steady environment. The mineral content of the soil and its heartiful structure are important for their well-being, but it is the life in the earth that...
- Terra pretaTerra pretaTerra preta is a type of very dark, fertile anthropogenic soil found in the Amazon Basin. Terra preta owes its name to its very high charcoal content, and was indeed made by adding a mixture of charcoal, bone, and manure to the otherwise relatively infertile Amazonian soil, and stays there for...
- FertilizerFertilizerFertilizer is any organic or inorganic material of natural or synthetic origin that is added to a soil to supply one or more plant nutrients essential to the growth of plants. A recent assessment found that about 40 to 60% of crop yields are attributable to commercial fertilizer use...

