
Fraunhofer lines
Encyclopedia
In physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
and optics
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
, the Fraunhofer lines are a set of spectral line
Spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...
s named for the German physicist Joseph von Fraunhofer
Joseph von Fraunhofer
Joseph von Fraunhofer was a German optician. He is known for the discovery of the dark absorption lines known as Fraunhofer lines in the Sun's spectrum, and for making excellent optical glass and achromatic telescope objectives.-Biography:Fraunhofer was born in Straubing, Bavaria...
(1787–1826). The lines were originally observed as dark features (absorption lines) in the optical spectrum of the Sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
.
The English chemist William Hyde Wollaston
William Hyde Wollaston
William Hyde Wollaston FRS was an English chemist and physicist who is famous for discovering two chemical elements and for developing a way to process platinum ore.-Biography:...
was in 1802 the first person to note the appearance of a number of dark features in the solar spectrum. In 1814, Fraunhofer independently rediscovered the lines and began a systematic study and careful measurement of the wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
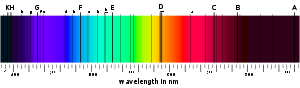
of these features. In all, he mapped over 570 lines, and designated the principal features with the letters A through K, and weaker lines with other letters. Modern observations of sunlight
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
can detect many thousands of lines.
About 45 years later Kirchhoff
Gustav Kirchhoff
Gustav Robert Kirchhoff was a German physicist who contributed to the fundamental understanding of electrical circuits, spectroscopy, and the emission of black-body radiation by heated objects...
and Bunsen
Robert Bunsen
Robert Wilhelm Eberhard Bunsen was a German chemist. He investigated emission spectra of heated elements, and discovered caesium and rubidium with Gustav Kirchhoff. Bunsen developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organoarsenic...
noticed that several Fraunhofer lines coincide with characteristic emission lines
Emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state....
identified in the spectra of heated elements. It was correctly deduced that dark lines in the solar spectrum are caused by absorption by chemical elements in the Solar atmosphere. Some of the observed features were identified as telluric lines
Telluric contamination
Telluric contamination is contamination of the astronomical spectra by the Earth's atmosphere.-Interference with astronomical observations:Most astronomical observations are conducted by measuring photons which originate beyond the sky...
originating from absorption in oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
molecules in the Earth's atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
.
The Fraunhofer lines are typical spectral absorption lines. These dark lines are produced whenever a cold gas is between a broad spectrum photon source and the detector. In this case a decrease in the intensity of light in the frequency of the incident photon is seen as the photons are absorbed, then re-emitted in random directions, which are mostly in directions different from the original one. This results in an absorption line, since the narrow frequency band of light initially traveling toward the detector, has been effectively scattered in other directions. Absorption lines are produced even during reflection from an illuminated cold gas, since after reflection there is still the opportunity for a selective absorption (and re-scatter) between the point of reflection and the detector. By contrast, if the detector sees photons emitted directly from a glowing gas, then the detector often sees photons emitted in a narrow frequency range by quantum emission processes in atoms in the hot gas, resulting in an emission line. In the Sun, Fraunhofer lines are seen from gas in the outer regions of the Sun, which are too cold to directly produce emission lines of the elements they represent.
The major Fraunhofer lines, and the elements they are associated with, are shown in the following table:
| Designation | Element | Wavelength (nm) | Designation | Element | Wavelength (nm) |
| y | O2 Oxygen Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition... |
898.765 | c | Fe | 495.761 |
| Z | O2 | 822.696 | F | Hβ | 486.134 |
| A | O2 | 759.370 | d | Fe | 466.814 |
| B | O2 | 686.719 | e | Fe | 438.355 |
| C | H Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... α |
656.281 | G' | Hγ | 434.047 |
| a | O2 | 627.661 | G | Fe | 430.790 |
| D1 | Na Sodium Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride... |
589.592 | G | Ca Calcium Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust... |
430.774 |
| D2 | Na | 588.995 | h | Hδ | 410.175 |
| D3 or d | He Helium Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table... |
587.5618 | H | Ca+ | 396.847 |
| e | Hg Mercury (element) Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum... |
546.073 | K | Ca+ | 393.368 |
| E2 | Fe Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... |
527.039 | L | Fe | 382.044 |
| b1 | Mg Magnesium Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole... |
518.362 | N | Fe | 358.121 |
| b2 | Mg | 517.270 | P | Ti Titanium Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color.... + |
336.112 |
| b3 | Fe | 516.891 | T | Fe | 302.108 |
| b4 | Fe | 516.891 | t | Ni Nickel Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile... |
299.444 |
| b4 | Mg | 516.733 | |||
The Fraunhofer C, F, G', and h lines correspond to the alpha, beta, gamma and delta lines of the Balmer series
Balmer series
The Balmer series or Balmer lines in atomic physics, is the designation of one of a set of six different named series describing the spectral line emissions of the hydrogen atom....
of emission lines of the hydrogen atom. The D1 and D2 lines form the well-known "sodium doublet", the centre wavelength of which (589.29 nm) is given the designation letter "D". This historical designation for this line has stuck and is given to the all the transitions between the ground state and the first excited state of the other alkali atoms as well. The D1 and D2 lines correspond to the fine splitting of the excited states. This may be confusing because the excited state for this transition is the P-state of the alkali and should not be confused with the higher D-states.
Note that there is disagreement in the literature for some line designations; e.g., the Fraunhofer d-line may refer to the cyan
Cyan
Cyan from , transliterated: kýanos, meaning "dark blue substance") may be used as the name of any of a number of colors in the blue/green range of the spectrum. In reference to the visible spectrum cyan is used to refer to the color obtained by mixing equal amounts of green and blue light or the...
iron line at 466.814 nm, or alternatively to the yellow
Yellow
Yellow is the color evoked by light that stimulates both the L and M cone cells of the retina about equally, with no significant stimulation of the S cone cells. Light with a wavelength of 570–590 nm is yellow, as is light with a suitable mixture of red and green...
helium line (also labeled D3) at 587.5618 nm. Similarly, there is ambiguity with reference to the e-line, since it can refer to the spectral lines of both iron (Fe) and mercury (Hg). In order to resolve ambiguities that arise in usage, ambiguous Fraunhofer line designations are preceded by the element with which they are associated (e.g., Mercury e-line and Helium d-line).
Because of their well defined wavelengths, Fraunhofer lines are often used to characterize the refractive index
Refractive index
In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
and dispersion
Dispersion (optics)
In optics, dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency, or alternatively when the group velocity depends on the frequency.Media having such a property are termed dispersive media...
properties of optical materials.

