Friedländer synthesis
Encyclopedia
The Friedländer synthesis is the chemical reaction
of 2-aminobenzaldehydes with ketone
s to form quinoline
derivatives. It is named after German chemist Paul Friedländer
(1857-1923).
This reaction has been catalyzed by trifluoroacetic acid
, toluenesulfonic acid, iodine
, and Lewis acid
s.
Several reviews have been published.
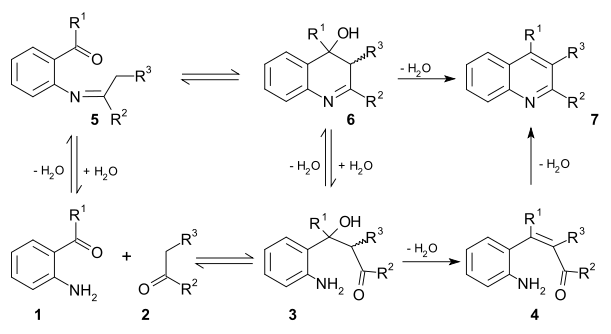
s exist for this reaction. In the first mechanism 2-amino substituted carbonyl compound 1 and carbonyl compound 2 react in a rate-limiting step to aldol
adduct 3. This intermediate loses water in an elimination reaction
to unsaturated carbonyl compound 4 and then loses water again in imine
formation to quinoline 7. In the second mechanism the first step is Schiff base
formation to 5 followed by Aldol reaction to 6 and elimination to 7 .
The Pfitzinger reaction
and the Niementowski quinoline synthesis
are variations.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of 2-aminobenzaldehydes with ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s to form quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
derivatives. It is named after German chemist Paul Friedländer
Paul Friedländer (chemist)
Paul Friedländer was a German chemist best known for his research on derivates of indigo and isolation of Tyrian purple from Murex brandaris.-Life and work:...
(1857-1923).
This reaction has been catalyzed by trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
, toluenesulfonic acid, iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, and Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s.
Several reviews have been published.
Mechanism
Two viable reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
s exist for this reaction. In the first mechanism 2-amino substituted carbonyl compound 1 and carbonyl compound 2 react in a rate-limiting step to aldol
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
adduct 3. This intermediate loses water in an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
to unsaturated carbonyl compound 4 and then loses water again in imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
formation to quinoline 7. In the second mechanism the first step is Schiff base
Schiff base
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
formation to 5 followed by Aldol reaction to 6 and elimination to 7 .
The Pfitzinger reaction
Pfitzinger reaction
The Pfitzinger reaction is the chemical reaction of isatin with base and a carbonyl compound to yield substituted quinoline-4-carboxylic acids.Several reviews have been published.-Reaction mechanism:...
and the Niementowski quinoline synthesis
Niementowski quinoline synthesis
The Niementowski quinoline synthesis is the chemical reaction of anthranilic acids and ketones to form γ-hydroxyquinoline derivatives.Several reviews have been published.-See also:* Camps quinoline synthesis* Friedländer synthesis...
are variations.
See also
- Doebner-Miller reactionDoebner-Miller reactionThe Doebner-Miller reaction is the organic reaction of an aniline with α,β-unsaturated carbonyl compounds to form quinolines.This reaction is also known as the Skraup-Doebner-Von Miller quinoline synthesis, and is named after the Czech chemist Zdenko Hans Skraup , and the Germans Oscar Döbner and...
- Povarov reactionPovarov reactionThe Povarov reaction is an organic reaction described as a formal cycloaddition between an aromatic imine and an alkene. The imine in this organic reaction is a condensation reaction product from an aniline type compound and a benzaldehyde type compound . The alkene must be electron rich which...
- Skraup reactionSkraup reactionThe Skraup synthesis is a chemical reaction used to synthesize quinolines. It is named after the Czech chemist Zdenko Hans Skraup . In the archetypal Skraup, aniline is heated with sulfuric acid, glycerol, and an oxidizing agent, like nitrobenzene to yield quinoline.In this example, nitrobenzene...