Glatiramer acetate
Encyclopedia
Glatiramer acetate is an immunomodulator
drug currently used to treat multiple sclerosis
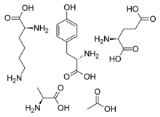
. It is a random polymer of four amino acids found in myelin basic protein
, namely glutamic acid
, lysine
, alanine
, and tyrosine
, and may work as a decoy for the immune system.
Glatiramer acetate is approved by the FDA for reducing the frequency of relapses, but not for reducing the progression of disability. Observational studies, but not randomized controlled trials, suggest that it may reduce progression of disability.
Although the clinical definition of multiple sclerosis requires two or more episodes of symptoms and signs, glatiramer acetate is approved for treatment after single episodes. It is also used to treat relapsing-remitting multiple sclerosis. It is administered by subcutaneous injection.
. The mechanism of action for glatiramer is unknown, although several have been proposed. Administration of glatiramer shifts the population of T cells from pro-inflammatory Th1 cells to regulatory Th2 cells that suppress the inflammatory response. Given its resemblance to myelin basic protein, glatiramer may also act as a sort of decoy, diverting an autoimmune response against myelin. The integrity of the blood-brain barrier, however, is not appreciably affected by glatiramer, at least not in the early stages of treatment. Glatiramer acetate has been shown in clinical trials to reduce the number and severity of exacerbations.
The mechanism(s) by which glatiramer acetate exerts its effects in patients with Multiple Sclerosis (MS) is (are) not fully elucidated. However, it is thought to act by modifying immune processes that are currently believed to be responsible for the pathogenesis of MS. This hypothesis is supported by findings of studies that have been carried out to explore the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a condition induced in several animal species through immunization against central nervous system derived material containing myelin and often used as an experimental animal model of MS. Studies in animals and in vitro systems suggest that upon its administration, glatiramer acetate-specific suppressor T-cells are induced and activated in the periphery.
In its pivotal trial of 251 patients, after 2 years Copaxone failed to show any advantage in halting disability progression.
As a result, Copaxane is approved by the FDA for reducing the frequency of relapses, but not for reducing the progression of disability.
A 15-year followup of the original trial compared patients who continued with glatiramir to patients who dropped out of the trial. Patients with glatiramer had reduced relapse rates, and decreased disability progression and transition to secondary progressive MS, compared to patients who did not continue glatiramer. However, the two groups were not necessarily comparable, as it was no longer a randomized trial. There were no long-term safety issues.
In 2 recent studies, both reported at the 2007 ECTRIMS meeting, the efficacy of glatiramer acetate was compared to high-dose/high-frequency interferon beta. In the REGARD study, Rebif was compared to glatiramer, and in the BEYOND study, Betaseron was compared to glatiramer. In both trials, there was no significant difference between interferon and glatiramer in the primary endpoints (time to relapse) or in any clinical endpoints, although some differences in MRI measures of disease activity have been claimed.
A double-blind 3-year study found no effect of glatiramer acetate on Primary-Progressive Multiple Sclerosis.
Copaxone has FDA approval for clinically isolated syndrome, based on the PreCISe trial, which showed that glatiramer delayed the progression from the first clinical event to clinically definite multiple sclerosis with a risk reduction of 45%. 43% of patients in the placebo group converted, compared to 25% in the glatiramer group.
in Rehovot, Israel. The efficacy and safety of glatiramer acetate were demonstrated in three main clinical trials. The first trial, led by Professor Murray Bornstein
, was performed in a single center, double-blind, placebo controlled trial
and included 50 patients.
The second trial was a 2-year, multi-center, randomized, double-blind, placebo controlled trial and was performed in eleven US centers involving 251 patients. This study was led by Professor Kenneth Johnson, Chairman of the Department of Neurology, University of Maryland Medical Center
, Baltimore
. The third trial, a double-blind, multi-center, multi-country MRI study, involved 29 MS Centers in six European countries and Canada
, with the participation of 239 patients. This study was led by Professor G. Comi, Department of Neuroscience, San Raffaele Hospital
, the University of Milan
.
, Israel
, Canada
and 24 European Union
Countries.
Approval in the US was obtained in 1996. Glatiramer acetate was approved for marketing in the U.K. in August 2000, and launched in December. This first approval in a major European market enabled Teva to file for approval all over the European Union
under the mutual recognition procedure.
 According to MediGuard
According to MediGuard
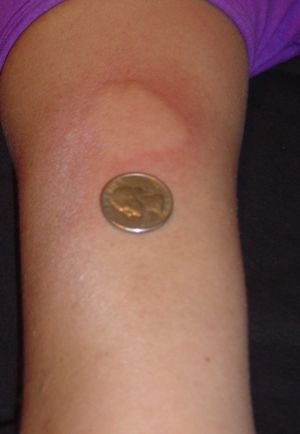
, side effects may include a lump at the injection site (injection site reaction) in approximately 30% of users, and aches, fever, chills (flu-like symptoms) in approximately 10% of users. Copaxone is the only disease modifying drug that does not cause flu like symptoms in patients. Side effect symptoms are generally mild in nature. A reaction that involves flushing, shortness in breath, anxiety & rapid heartbeat has been reported soon after injection in up to 5% of patients (usually after injecting directly into a vein). These side effects subside within thirty minutes. Over time, a visible dent at the injection site can occur due to the local destruction of fat tissue, known as lipoatrophy
, that may develop.
More serious side effects have been reported for glatiramer acetate, according to the FDA's prescribing label, these include serious side effects to the body's Cardiovascular System, Digestive System (including Liver),
Hemic and Lymphatic System, Musculoskeletal System, Nervous System, Respiratory System, Special Senses (in particular the eyes), Urogenital System; also reported have been Metabolic and Nutritional Disorders; however a link between glatiramer acetate and these adverse effects has not been definitively established.
.
Glatiramer is currently in Phase I clinical trials for Dry Age-Related Macular Degeneration (AMD).
Immunomodulator
An immunomodulator, also known as an immunotherapy is a substance which has an effect on the immune system.- Immunosuppressants :Inhibits immune response in organ transplantation and autoimmune diseases.- Immunostimulants :...
drug currently used to treat multiple sclerosis
Multiple sclerosis
Multiple sclerosis is an inflammatory disease in which the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, leading to demyelination and scarring as well as a broad spectrum of signs and symptoms...
. It is a random polymer of four amino acids found in myelin basic protein
Myelin basic protein
Myelin basic protein is a protein believed to be important in the process of myelination of nerves in the central nervous system .MBP was initially sequenced in 1971 after isolation from myelin membranes...
, namely glutamic acid
Glutamic acid
Glutamic acid is one of the 20 proteinogenic amino acids, and its codons are GAA and GAG. It is a non-essential amino acid. The carboxylate anions and salts of glutamic acid are known as glutamates...
, lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
, alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
, and tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, and may work as a decoy for the immune system.
Glatiramer acetate is approved by the FDA for reducing the frequency of relapses, but not for reducing the progression of disability. Observational studies, but not randomized controlled trials, suggest that it may reduce progression of disability.
Although the clinical definition of multiple sclerosis requires two or more episodes of symptoms and signs, glatiramer acetate is approved for treatment after single episodes. It is also used to treat relapsing-remitting multiple sclerosis. It is administered by subcutaneous injection.
Mechanism of action
Glatiramer acetate is a random polymer (average molecular mass 6.4 kD) composed of four amino acids that are found in myelin basic proteinMyelin basic protein
Myelin basic protein is a protein believed to be important in the process of myelination of nerves in the central nervous system .MBP was initially sequenced in 1971 after isolation from myelin membranes...
. The mechanism of action for glatiramer is unknown, although several have been proposed. Administration of glatiramer shifts the population of T cells from pro-inflammatory Th1 cells to regulatory Th2 cells that suppress the inflammatory response. Given its resemblance to myelin basic protein, glatiramer may also act as a sort of decoy, diverting an autoimmune response against myelin. The integrity of the blood-brain barrier, however, is not appreciably affected by glatiramer, at least not in the early stages of treatment. Glatiramer acetate has been shown in clinical trials to reduce the number and severity of exacerbations.
The mechanism(s) by which glatiramer acetate exerts its effects in patients with Multiple Sclerosis (MS) is (are) not fully elucidated. However, it is thought to act by modifying immune processes that are currently believed to be responsible for the pathogenesis of MS. This hypothesis is supported by findings of studies that have been carried out to explore the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a condition induced in several animal species through immunization against central nervous system derived material containing myelin and often used as an experimental animal model of MS. Studies in animals and in vitro systems suggest that upon its administration, glatiramer acetate-specific suppressor T-cells are induced and activated in the periphery.
Effectiveness
A 2004 Cochrane review concluded that Glatiramer acetate "did not show any beneficial effect on the main outcome measures in MS, i.e. disease progression, and it does not substantially affect the risk of clinical relapses."In its pivotal trial of 251 patients, after 2 years Copaxone failed to show any advantage in halting disability progression.
As a result, Copaxane is approved by the FDA for reducing the frequency of relapses, but not for reducing the progression of disability.
A 15-year followup of the original trial compared patients who continued with glatiramir to patients who dropped out of the trial. Patients with glatiramer had reduced relapse rates, and decreased disability progression and transition to secondary progressive MS, compared to patients who did not continue glatiramer. However, the two groups were not necessarily comparable, as it was no longer a randomized trial. There were no long-term safety issues.
In 2 recent studies, both reported at the 2007 ECTRIMS meeting, the efficacy of glatiramer acetate was compared to high-dose/high-frequency interferon beta. In the REGARD study, Rebif was compared to glatiramer, and in the BEYOND study, Betaseron was compared to glatiramer. In both trials, there was no significant difference between interferon and glatiramer in the primary endpoints (time to relapse) or in any clinical endpoints, although some differences in MRI measures of disease activity have been claimed.
A double-blind 3-year study found no effect of glatiramer acetate on Primary-Progressive Multiple Sclerosis.
Copaxone has FDA approval for clinically isolated syndrome, based on the PreCISe trial, which showed that glatiramer delayed the progression from the first clinical event to clinically definite multiple sclerosis with a risk reduction of 45%. 43% of patients in the placebo group converted, compared to 25% in the glatiramer group.
Development
Glatiramer acetate was originally discovered by Dr. Dvora Teitelbaum at the Weizmann Institute of ScienceWeizmann Institute of Science
The Weizmann Institute of Science , known as Machon Weizmann, is a university and research institute in Rehovot, Israel. It differs from other Israeli universities in that it offers only graduate and post-graduate studies in the sciences....
in Rehovot, Israel. The efficacy and safety of glatiramer acetate were demonstrated in three main clinical trials. The first trial, led by Professor Murray Bornstein
Murray Bornstein
Dr. Murray B. Bornstein was an American neuroscientist and co-developer of the multiple sclerosis drug Copaxone.-References:...
, was performed in a single center, double-blind, placebo controlled trial
Placebo-controlled studies
A Placebo-controlled study is a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect...
and included 50 patients.
The second trial was a 2-year, multi-center, randomized, double-blind, placebo controlled trial and was performed in eleven US centers involving 251 patients. This study was led by Professor Kenneth Johnson, Chairman of the Department of Neurology, University of Maryland Medical Center
University of Maryland Medical Center
The University of Maryland Medical Center is a teaching hospital with 705 beds based in Baltimore, Maryland, that provides the full range of health care to people throughout Maryland and the Mid-Atlantic region. It gets more than 35,000 inpatient admissions and 165,000 outpatient visits each year...
, Baltimore
Baltimore
Baltimore is the largest independent city in the United States and the largest city and cultural center of the US state of Maryland. The city is located in central Maryland along the tidal portion of the Patapsco River, an arm of the Chesapeake Bay. Baltimore is sometimes referred to as Baltimore...
. The third trial, a double-blind, multi-center, multi-country MRI study, involved 29 MS Centers in six European countries and Canada
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
, with the participation of 239 patients. This study was led by Professor G. Comi, Department of Neuroscience, San Raffaele Hospital
San Raffaele Hospital
The San Raffaele Hospital is a university hospital situated in Milan, Italy.It was founded in 1969 by don Luigi Maria Verzé, president of "San Raffaele del Monte Tabor Foundation"....
, the University of Milan
University of Milan
The University of Milan is a higher education institution in Milan, Italy. It is one of the largest universities in Europe, with about 62,801 students, a teaching and research staff of 2,455 and a non-teaching staff of 2,200....
.
Marketing and distribution
Glatiramer acetate has been approved for marketing in 49 countries worldwide, including the United StatesUnited States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
, Israel
Israel
The State of Israel is a parliamentary republic located in the Middle East, along the eastern shore of the Mediterranean Sea...
, Canada
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
and 24 European Union
European Union
The European Union is an economic and political union of 27 independent member states which are located primarily in Europe. The EU traces its origins from the European Coal and Steel Community and the European Economic Community , formed by six countries in 1958...
Countries.
Approval in the US was obtained in 1996. Glatiramer acetate was approved for marketing in the U.K. in August 2000, and launched in December. This first approval in a major European market enabled Teva to file for approval all over the European Union
European Union
The European Union is an economic and political union of 27 independent member states which are located primarily in Europe. The EU traces its origins from the European Coal and Steel Community and the European Economic Community , formed by six countries in 1958...
under the mutual recognition procedure.
Side effects

IGuard
The holding company for MediGuard.org is iGuard Inc.. The aim of the company is to monitor the safety of prescription medicines, over-the-counter medicines and healthcare supplements. As of February 2011, there are over 2,480,000 registered users in the company's web-site...
, side effects may include a lump at the injection site (injection site reaction) in approximately 30% of users, and aches, fever, chills (flu-like symptoms) in approximately 10% of users. Copaxone is the only disease modifying drug that does not cause flu like symptoms in patients. Side effect symptoms are generally mild in nature. A reaction that involves flushing, shortness in breath, anxiety & rapid heartbeat has been reported soon after injection in up to 5% of patients (usually after injecting directly into a vein). These side effects subside within thirty minutes. Over time, a visible dent at the injection site can occur due to the local destruction of fat tissue, known as lipoatrophy
Lipoatrophy
Lipoatrophy is the term describing the localized loss of fat tissue. This may occur as a result of subcutanous injections of insulin in the treatment of diabetes, from the use of human growth hormone or from subcutanous injections of Copaxone used for the treatment of multiple sclerosis. In the...
, that may develop.
More serious side effects have been reported for glatiramer acetate, according to the FDA's prescribing label, these include serious side effects to the body's Cardiovascular System, Digestive System (including Liver),
Hemic and Lymphatic System, Musculoskeletal System, Nervous System, Respiratory System, Special Senses (in particular the eyes), Urogenital System; also reported have been Metabolic and Nutritional Disorders; however a link between glatiramer acetate and these adverse effects has not been definitively established.
Research
Glatiramer has been found to be protective in a mouse model of cerebral malariaMalaria
Malaria is a mosquito-borne infectious disease of humans and other animals caused by eukaryotic protists of the genus Plasmodium. The disease results from the multiplication of Plasmodium parasites within red blood cells, causing symptoms that typically include fever and headache, in severe cases...
.
Glatiramer is currently in Phase I clinical trials for Dry Age-Related Macular Degeneration (AMD).

