_fluoride.gif)
Gold(III) fluoride
Encyclopedia
Gold fluoride, , is an orange solid that sublimes at 300 °C. It is a powerful fluorinating agent
.
with F2
or BrF3
.
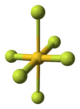
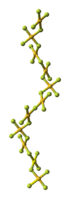
of AuF3 consists of spirals of square-planar
AuF4 units.
Halogenation
Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination...
.
Preparation
AuF3 can be prepared by reacting AuCl3Gold(III) chloride
Gold chloride, traditionally called auric chloride, is a chemical compound of gold and chlorine. With the molecular formula Au2Cl6, the name gold trichloride is a simplification, referring to the empirical formula. The Roman numerals in the name indicate that the gold has an oxidation state of +3,...
with F2
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
or BrF3
Bromine trifluoride
Bromine trifluoride is an interhalogen compound with the formula BrF3. This toxic, colourless, and corrosive liquid is soluble in sulfuric acid but explodes on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent...
.
Structure
The crystal structureCrystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
of AuF3 consists of spirals of square-planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
AuF4 units.
 |
 |
 |
 |
|

