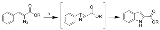
Hemetsberger indole synthesis
Encyclopedia
The Hemetsberger indole synthesis (also called the Hemetsberger-Knittel synthesis) is a chemical reaction
that thermally decomposes a 3-aryl-2-azido-propenoic ester into an indole
-2-carboxylic ester
.
 Yields are typically above 70%. However, this is not a popular reaction, due to the lack of stability and difficulty in synthesizing the starting material.
Yields are typically above 70%. However, this is not a popular reaction, due to the lack of stability and difficulty in synthesizing the starting material.
intermediate.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that thermally decomposes a 3-aryl-2-azido-propenoic ester into an indole
Indole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
-2-carboxylic ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
.

Reaction mechanism
The mechanism is unknown. However, aziridine intermediates have been isolated. The mechanism is postulated to proceed via a nitreneNitrene
In chemistry, a nitrene is the nitrogen analogue of a carbene. The nitrogen atom has only 6 valence electrons and is therefore considered an electrophile...
intermediate.

