
Homogeneous catalysis
Encyclopedia
In chemistry
, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent
with the reactants.
In an illustrative case, acids accelerate (catalyse) the hydrolysis
of ester
s:
In the absence of acids, aqueous solutions of most esters do not hydrolyze at practical rates.
. Some well-known examples of homogeneous catalysis include hydroformylation
and transfer hydrogenation
, as well as certain kinds of Ziegler-Natta polymerization and hydrogenation
.
Many non-organometallic complexes are also widely used in catalysis, e.g. for the production of terephthalic acid
from xylene
.
s are homogeneous catalysts that are essential for life but are also harnessed for industrial processes. A well studied example carbonic anhydride, which catalyzes the release of CO2 into the lungs from the blood stream.
, where the catalyst is in a different phase than the reactants. One example of heterogeneous catalysis is the petrochemical alkylation
process, where the liquid reactants are immiscible with a solution containing the catalyst. Heterogeneous catalysis offers the advantage that products are readily separated from the catalyst, and heterogeneous catalysts are often more stable and degrade much slower than homogeneous catalysts. However, heterogeneous catalysts are difficult to study, so their reaction mechanisms are often unknown.
Enzyme
s possess properties of both homogeneous and heterogeneous catalysts. As such, they are usually regarded as a third, separate category of catalyst.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
with the reactants.
Acid catalysis
The proton is the most pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of waterSelf-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
In an illustrative case, acids accelerate (catalyse) the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s:
- CH3CO2CH3 + H2O
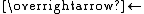 CH3CO2H + CH3OH
CH3CO2H + CH3OH
In the absence of acids, aqueous solutions of most esters do not hydrolyze at practical rates.
Organometallic chemistry
Processes that utilize soluble organometallic compounds as catalysts fall under the category of homogenous catalysis, as opposed to processes that use bulk metal or metal on a solid support which are examples of heterogeneous catalysisHeterogeneous catalysis
In chemistry, heterogeneous catalysis refers to the form of catalysis where the phase of the catalyst differs from that of the reactants. Phase here refers not only to solid, liquid, vs gas, but also immiscible liquids, e.g. oil and water. The great majority of practical heterogeneous catalysts...
. Some well-known examples of homogeneous catalysis include hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
and transfer hydrogenation
Transfer hydrogenation
Transfer hydrogenation is the addition of hydrogen to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2...
, as well as certain kinds of Ziegler-Natta polymerization and hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
.
Many non-organometallic complexes are also widely used in catalysis, e.g. for the production of terephthalic acid
Terephthalic acid
Terephthalic acid is the organic compound with formula C6H42. This colourless solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several billion kilograms are produced annually...
from xylene
Xylene
Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
.
Other forms of homogeneous catalysis
EnzymeEnzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s are homogeneous catalysts that are essential for life but are also harnessed for industrial processes. A well studied example carbonic anhydride, which catalyzes the release of CO2 into the lungs from the blood stream.
Contrast with heterogeneous catalysis
Homogeneous catalysis is the opposite of heterogeneous catalysisHeterogeneous catalysis
In chemistry, heterogeneous catalysis refers to the form of catalysis where the phase of the catalyst differs from that of the reactants. Phase here refers not only to solid, liquid, vs gas, but also immiscible liquids, e.g. oil and water. The great majority of practical heterogeneous catalysts...
, where the catalyst is in a different phase than the reactants. One example of heterogeneous catalysis is the petrochemical alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
process, where the liquid reactants are immiscible with a solution containing the catalyst. Heterogeneous catalysis offers the advantage that products are readily separated from the catalyst, and heterogeneous catalysts are often more stable and degrade much slower than homogeneous catalysts. However, heterogeneous catalysts are difficult to study, so their reaction mechanisms are often unknown.
Enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s possess properties of both homogeneous and heterogeneous catalysts. As such, they are usually regarded as a third, separate category of catalyst.

