
Human impacts on the nitrogen cycle
Encyclopedia
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
(N) inputs to the environment currently exceed inputs from natural N fixation. As a consequence of anthropogenic inputs, the global nitrogen cycle
Nitrogen cycle
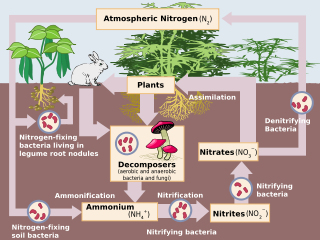
The nitrogen cycle is the process by which nitrogen is converted between its various chemical forms. This transformation can be carried out by both biological and non-biological processes. Important processes in the nitrogen cycle include fixation, mineralization, nitrification, and denitrification...
(Fig. 1) has been significantly altered over the past century. Global atmospheric nitrous oxide
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
(N2O) mole fractions have increased from a pre-industrial value of ~270 nmol/mol to ~319 nmol/mol in 2005. Human activities account for over one-third of N2O emissions, most of which are due to the agricultural sector. This article is intended to give a brief review of the history of anthropogenic N inputs, and reported impacts of nitrogen inputs on selected terrestrial and aquatic ecosystems.
History of anthropogenic nitrogen inputs
Approximately 78% of earth’s atmosphere is N gas (N2), which is an inert compound and biologically unavailable to most organisms. In order to be utilized in most biological processes, N2 must be converted to reactive N (Nr), which includes inorganic reducedforms (NH3 and NH4+), inorganic oxidized forms (NO, NO2, HNO3, N2O, and NO3-), and organic compounds (urea, amines, and proteins).
N2 has a strong triple bond, and so a significant amount of energy (226 kcal mol-1) is required to convert N2 to Nr. Prior to industrial processes, the only sources of such energy were solar radiation and electrical discharges. Utilizing a large amount of metabolic energy and the enzyme nitrogenase
Nitrogenase
Nitrogenases are enzymes used by some organisms to fix atmospheric nitrogen gas . It is the only known family of enzymes that accomplish this process. Dinitrogen is quite inert because of the strength of its N-N triple bond...
, some bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and cyanobacteria convert atmospheric N2 to NH3, a process known as biological nitrogen fixation
Nitrogen fixation
Nitrogen fixation is the natural process, either biological or abiotic, by which nitrogen in the atmosphere is converted into ammonia . This process is essential for life because fixed nitrogen is required to biosynthesize the basic building blocks of life, e.g., nucleotides for DNA and RNA and...
(BNF). The anthropogenic analogue to BNF is the Haber-Bosch process, in which fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
H2 is reacted with atmospheric N2 at high temperatures and pressures to produce NH3. Lastly, N2 is converted to NO by energy from lightning
Lightning
Lightning is an atmospheric electrostatic discharge accompanied by thunder, which typically occurs during thunderstorms, and sometimes during volcanic eruptions or dust storms...
, which is negligible in current temperate ecosystems, or by fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
combustion.
Until 1850, natural BNF, cultivation-induced BNF (e.g., planting of leguminous crops), and incorporated organic matter were the only sources of N for agricultural production. Near the turn of the century, Nr from guano
Guano
Guano is the excrement of seabirds, cave dwelling bats, and seals. Guano manure is an effective fertilizer due to its high levels of phosphorus and nitrogen and also its lack of odor. It was an important source of nitrates for gunpowder...
and sodium nitrate
Sodium nitrate
Sodium nitrate is the chemical compound with the formula NaNO3. This salt, also known as Chile saltpeter or Peru saltpeter to distinguish it from ordinary saltpeter, potassium nitrate, is a white solid which is very soluble in water...
deposits was harvested and exported from the arid Pacific islands and South American deserts. By the late 1920s, early industrial processes, albeit inefficient, were commonly used to produce NH3. Due to the efforts of Fritz Haber
Fritz Haber
Fritz Haber was a German chemist, who received the Nobel Prize in Chemistry in 1918 for his development for synthesizing ammonia, important for fertilizers and explosives. Haber, along with Max Born, proposed the Born–Haber cycle as a method for evaluating the lattice energy of an ionic solid...
and Carl Bosch
Carl Bosch
Carl Bosch was a German chemist and engineer and Nobel laureate in chemistry. He was a pioneer in the field of high-pressure industrial chemistry and founder of IG Farben, at one point the world's largest chemical company....
, the Haber-Bosch process became the largest source of nitrogenous fertilizer after the 1950s, and replaced BNF as the dominant source of NH3 production. From 1890 to 1990, anthropogenically created Nr increased almost ninefold. During this time, global population more than tripled, partly due to increased food production.
Since the industrial revolution
Industrial Revolution
The Industrial Revolution was a period from the 18th to the 19th century where major changes in agriculture, manufacturing, mining, transportation, and technology had a profound effect on the social, economic and cultural conditions of the times...
, an additional source of anthropogenic N input has been fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
combustion, which is used to generate energy (e.g., to power automobiles). During combustion of fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
s, high temperatures and pressures provide energy to produce NO from N2 oxidation. Additionally, when fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
is extracted and burned, fossil N may become reactive (i.e., NOx emissions). During the 1970s, scientists began to recognize that N inputs were accumulating in the environment and affecting ecosystem functioning.
Impacts of anthropogenic inputs on the nitrogen cycle
Between 1600 and 1990, global reactive nitrogen (Nr) creation had increased nearly 50%. During this period, atmospheric emissions of Nr species reportedly increased 250% and deposition to marine and terrestrial ecosystems increased over 200%. Additionally, there was a reported fourfold increase in riverine dissolved inorganic N fluxes to coasts. N is a critical limiting nutrient in many systems, including forests, wetlands, and coastal and marine ecosystems; therefore, this change in emissions and distribution of Nr has resulted in substantial consequences for aquatic and terrestrial ecosystems.Atmosphere
Atmospheric Nr inputs mainly include oxides of N (NOx), ammonia (NH3), and nitrous oxide (N2O) from aquatic and terrestrial ecosystems, and NOx from fossil fuelFossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
and biomass combustion.
In agroecosystem
Agroecosystem
An agroecosystem is the basic unit of study for an agroecologist, and is somewhat arbitrarily defined as a spatially and functionally coherent unit of agricultural activity, and includes the living and nonliving components involved in that unit as well as their interactions.An agroecosystem can be...
s, fertilizer application has increased microbial nitrification
Nitrification
Nitrification is the biological oxidation of ammonia with oxygen into nitrite followed by the oxidation of these nitrites into nitrates. Degradation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil...
(aerobic process in which microorganisms oxidize ammonium [NH4+] to nitrate [NO3-]) and denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
(anaerobic process in which microorganisms reduce NO3- to atmospheric nitrogen gas [N2]). Both processes naturally leak nitric oxide (NO) and nitrous oxide (N2O) to the atmosphere. Of particular concern is N2O, which has an average atmospheric lifetime of 114–120 years, and is 300 times more effective than CO2 as a greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
. NOx produced by industrial processes, automobiles and agricultural fertilization and NH3 emitted from soils (i.e., as an additional byproduct of nitrification) and livestock operations are transported to downwind ecosystems, influencing N cycling and nutrient losses. Six major effects of NOx and NH3 emissions have been cited: 1) decreased atmospheric visibility due to ammonium aerosols (fine particulate matter [PM]); 2) elevated ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
concentrations; 3) ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
and PM affects human health (e.g. respiratory disease
Respiratory disease
Respiratory disease is a medical term that encompasses pathological conditions affecting the organs and tissues that make gas exchange possible in higher organisms, and includes conditions of the upper respiratory tract, trachea, bronchi, bronchioles, alveoli, pleura and pleural cavity, and the...
s, cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
); 4) increases in relative forcing and
global climate change; 5) decreased agricultural productivity due to ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
deposition; and 6) ecosystem acidification and eutrophication
Eutrophication
Eutrophication or more precisely hypertrophication, is the movement of a body of water′s trophic status in the direction of increasing plant biomass, by the addition of artificial or natural substances, such as nitrates and phosphates, through fertilizers or sewage, to an aquatic system...
.
Biosphere
Terrestrial and aquatic ecosystems receive Nr inputs from the atmosphere through wet and dry deposition. Atmospheric Nr species can be deposited to ecosystems in precipitation (e.g., NO3-, NH4+, organic N compounds), as gases (e.g., NH3 and gaseous nitric acid [HNO3]), or as aerosols (e.g., ammonium nitrate [NH4NO3]). Aquatic ecosystems receive additional nitrogen from surface runoffSurface runoff
Surface runoff is the water flow that occurs when soil is infiltrated to full capacity and excess water from rain, meltwater, or other sources flows over the land. This is a major component of the water cycle. Runoff that occurs on surfaces before reaching a channel is also called a nonpoint source...
and river
River
A river is a natural watercourse, usually freshwater, flowing towards an ocean, a lake, a sea, or another river. In a few cases, a river simply flows into the ground or dries up completely before reaching another body of water. Small rivers may also be called by several other names, including...
ine inputs.
Increased N deposition can acidify soils, streams, and lakes and alter forest and grassland productivity. In forest and grassland ecosystems, Nr inputs have produced initial increases in productivity followed by declines as critical thresholds are exceeded. Nr effects on biodiversity
Biodiversity
Biodiversity is the degree of variation of life forms within a given ecosystem, biome, or an entire planet. Biodiversity is a measure of the health of ecosystems. Biodiversity is in part a function of climate. In terrestrial habitats, tropical regions are typically rich whereas polar regions...
, carbon cycling, and changes in species composition have also been demonstrated. In highly developed areas of near shore coastal ocean and estuarine systems, rivers deliver direct (e.g., surface runoff
Surface runoff
Surface runoff is the water flow that occurs when soil is infiltrated to full capacity and excess water from rain, meltwater, or other sources flows over the land. This is a major component of the water cycle. Runoff that occurs on surfaces before reaching a channel is also called a nonpoint source...
) and indirect (e.g., groundwater contamination) N inputs from agroecosystems. Increased N inputs can result in freshwater acidification and eutrophication
Eutrophication
Eutrophication or more precisely hypertrophication, is the movement of a body of water′s trophic status in the direction of increasing plant biomass, by the addition of artificial or natural substances, such as nitrates and phosphates, through fertilizers or sewage, to an aquatic system...
of marine waters.
Impacts on productivity and nutrient cycling
Much of terrestrial growth in temperate systems is limited by N; therefore, N inputs (i.e., through deposition and fertilization) can increase N availability, which temporarily increases N uptake, plant and microbial growth, and N accumulation in plant biomass and soil organic matter. Incorporation of greater amounts of N in organic matter decreases C:N ratios, increasing mineral N release (NH4+) during organic matter decomposition by heterotrophic microbes (i.e.ammonification). As ammonification increases, so does nitrification of the mineralized N. Because microbial nitrification and denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
are “leaky”, N deposition is expected to increase trace gas emissions. Additionally, with increasing NH4+ accumulation in the soil, nitrification processes release hydrogen ions, which acidify the soil. NO3-, the product of nitrification, is highly mobile and can be leached from the soil, along with positively charged alkaline minerals such as calcium and magnesium. In acid soils, mobilized aluminum ions can reach toxic concentrations, negatively affecting both terrestrial and adjacent aquatic ecosystems.
Anthropogenic sources of N generally reach upland forests through deposition
Deposition (Aerosol physics)
In aerosol physics, Deposition is the process by which aerosol particles collect or deposit themselves on solid surfaces, decreasing the concentration of the particles in the air. It can be divided into two sub-processes: dry and wet deposition. The rate of deposition, or the deposition velocity,...
. A potential concern of increased N deposition due to human activities is altered nutrient cycling in forest ecosystems. Numerous studies have demonstrated both positive and negative impacts of atmospheric N deposition on forest productivity and carbon storage. Added N is often rapidly immobilized by microbes, and the effect of the remaining available N depends on the plant community’s capacity for N uptake. In systems with high uptake, N is assimilated into the plant biomass, leading to enhanced net primary productivity (NPP) and possibly increased carbon sequestration through greater photosynthetic capacity. However, ecosystem responses to N additions are contingent upon many site-specific factors including climate, land-use history, and amount of N additions. For example, in the Northeastern United States, hardwood stands receiving chronic N inputs have demonstrated greater capacity to retain N and increase annual net primary productivity (ANPP) than conifer stands. Once N input exceeds system demand, N may be lost via leaching and gas fluxes. When available N exceeds the ecosystem’s (i.e., vegetation, soil, and microbes, etc.) uptake capacity, N saturation
Saturation
Saturation or saturated may refer to:- Meteorology :* Dew point, which is a temperature that occurs when atmospheric humidity reaches 100% and the air is saturated with moisture- Physics :...
occurs and excess N is lost to surface waters, groundwater, and the atmosphere. N saturation can result in nutrient imbalances (e.g., loss of calcium due to nitrate leaching) and possible forest decline.
A 15-year study of chronic N additions at the Harvard Forest Long Term Ecological Research (LTER) program has elucidated many impacts of increased nitrogen deposition on nutrient cycling in temperate forests. It found that chronic N additions resulted in greater leaching losses, increased pine mortality, and cessation of biomass accumulation. Another study reported that chronic N additions resulted in accumulation of non-photosynthetic N and subsequently reduced photosynthetic capacity, supposedly leading to severe carbon stress and mortality. These findings negate previous hypotheses that increased N inputs would increase NPP and carbon sequestration.
Impacts on plant species diversity
Many plant communities have evolved under low nutrient conditions; therefore, increased N inputs can alter biotic and abiotic interactions, leading to changes in community composition. Several nutrient addition studies have shown that increased N inputs lead to dominance of fast-growing plant species, with associated declines in species richness. Other studies have found that secondary responses of the system to N enrichment, including soil acidification
Soil acidification
Soil acidification is the buildup of hydrogen cations, also called protons, reducing the soil pH. This happens when a proton donor is added to the soil. The donor can be an acid, such as nitric acid and sulfuric acid . It can also be a compound such as aluminium sulfate, which reacts in the soil to...
and changes in mycorrhizal communities have allowed stress-tolerant species to out-compete sensitive species. Two other studies found evidence that increased N availability has resulted in declines in species-diverse heathlands. Heathlands are characterized by N-poor soils, which exclude N-demanding grasses; however, with increasing N deposition and soil acidification
Soil acidification
Soil acidification is the buildup of hydrogen cations, also called protons, reducing the soil pH. This happens when a proton donor is added to the soil. The donor can be an acid, such as nitric acid and sulfuric acid . It can also be a compound such as aluminium sulfate, which reacts in the soil to...
, invading grasslands replace lowland heath.
In a more recent experimental study of N fertilization and disturbance (i.e., tillage) in old field succession, it was found that species richness decreased with increasing N, regardless of disturbance level. Competition experiments showed that competitive dominants excluded competitively inferior species between disturbance events. With increased N inputs, competition shifted from belowground to aboveground (i.e., to competition for light), and patch colonization rates significantly decreased. These internal changes can dramatically affect the community by shifting the balance of competition-colonization tradeoffs between species. In patch-based systems, regional coexistence can occur through tradeoffs in competitive and colonizing abilities given sufficiently high disturbance rates. That is, with inverse ranking of competitive and colonizing abilities, plants can coexist in space and time as disturbance removes superior competitors from patches, allowing for establishment of superior colonizers. However, as demonstrated by Wilson and Tilman, increased nutrient inputs can negate tradeoffs, resulting in competitive exclusion of these superior colonizers/poor competitors.
Aquatic ecosystems
Aquatic ecosystems also exhibit varied responses to nitrogen enrichment. NO3- loading from N saturated, terrestrial ecosystems can lead to acidificationOcean acidification
Ocean acidification is the name given to the ongoing decrease in the pH and increase in acidity of the Earth's oceans, caused by the uptake of anthropogenic carbon dioxide from the atmosphere....
of downstream freshwater systems and eutrophication
Eutrophication
Eutrophication or more precisely hypertrophication, is the movement of a body of water′s trophic status in the direction of increasing plant biomass, by the addition of artificial or natural substances, such as nitrates and phosphates, through fertilizers or sewage, to an aquatic system...
of downstream marine systems. Freshwater acidification can cause aluminum toxicity and mortality of pH-sensitive fish species. Because marine systems are generally nitrogen-limited, excessive N inputs can result in water quality degradation due to toxic algal blooms, oxygen deficiency, habitat loss, decreases in biodiversity
Biodiversity
Biodiversity is the degree of variation of life forms within a given ecosystem, biome, or an entire planet. Biodiversity is a measure of the health of ecosystems. Biodiversity is in part a function of climate. In terrestrial habitats, tropical regions are typically rich whereas polar regions...
, and fishery losses.
Acidification of freshwaters
Atmospheric N deposition in terrestrial landscapes can be transformed through soil microbial processes to biologically available nitrogen, which can result in surface-water acidification, and loss of biodiversity
Biodiversity
Biodiversity is the degree of variation of life forms within a given ecosystem, biome, or an entire planet. Biodiversity is a measure of the health of ecosystems. Biodiversity is in part a function of climate. In terrestrial habitats, tropical regions are typically rich whereas polar regions...
. NO3- and NH4+ inputs from terrestrial systems and the atmosphere can acidify freshwater systems when there is little buffering capacity due to soil acidification
Soil acidification
Soil acidification is the buildup of hydrogen cations, also called protons, reducing the soil pH. This happens when a proton donor is added to the soil. The donor can be an acid, such as nitric acid and sulfuric acid . It can also be a compound such as aluminium sulfate, which reacts in the soil to...
. N pollution in Europe, the Northeastern United States, and Asia is a current concern for freshwater acidification. Lake acidification studies in the Experimental Lake Area (ELA) in northwestern Ontario clearly demonstrated the negative effects of increased acidity on a native fish species: lake trout (Salvelinus namaycush) recruitment and growth dramatically decreased due to extirpation of its key prey species during acidification.
Eutrophication of marine systems
Urbanization, deforestation, and agricultural activities largely contribute sediment and nutrient inputs to coastal waters via rivers. Increased nutrient inputs to marine systems have shown both short-term increases in productivity and fishery yields, and long-term detrimental effects of eutrophication
Eutrophication
Eutrophication or more precisely hypertrophication, is the movement of a body of water′s trophic status in the direction of increasing plant biomass, by the addition of artificial or natural substances, such as nitrates and phosphates, through fertilizers or sewage, to an aquatic system...
. Tripling of NO3- loads in the Mississippi River in the last half of the 20th century have been correlated with increased fishery yields in waters surrounding the Mississippi delta; however, these nutrient inputs have produced seasonal hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
(oxygen concentrations less than 2–3 mg L−1, "dead zone
Dead zone (ecology)
Dead zones are hypoxic areas in the world's oceans, the observed incidences of which have been increasing since oceanographers began noting them in the 1970s. These occur near inhabited coastlines, where aquatic life is most concentrated...
s") in the Gulf of Mexico
Gulf of Mexico
The Gulf of Mexico is a partially landlocked ocean basin largely surrounded by the North American continent and the island of Cuba. It is bounded on the northeast, north and northwest by the Gulf Coast of the United States, on the southwest and south by Mexico, and on the southeast by Cuba. In...
. In estuarine and coastal systems, high nutrient inputs increase primary production (e.g., phytoplankton, sea grasses, macroalgae), which increase turbidity
Turbidity
Turbidity is the cloudiness or haziness of a fluid caused by individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of water quality....
with resulting decreases in light penetration throughout the water column. Consequently, submerged vegetation growth declines, which reduces habitat complexity and oxygen production. The increased primary (i.e., phytoplankton, macroalgae, etc.) production leads to a flux of carbon to bottom waters when decaying organic matter (i.e., senescent primary production) sinks and is consumed by aerobic bacteria lower in the water column. As a result, oxygen consumption in bottom waters is greater than diffusion of oxygen from surface waters .
Integration
The above system responses to reactive nitrogen (Nr) inputs are almost all exclusively studied separately; however, research increasingly indicates that nitrogen loading problems are linked by multiple pathways transporting nutrients across system boundaries. This sequential transfer between ecosystems is termed the nitrogen cascade. (see illustration from United Nations Environment Programme). During the cascade, some systems accumulate Nr, which results in a time lag in the cascade and enhanced effects of Nr on the environment in which it accumulates. Ultimately, anthropogenic inputs of Nr are either accumulated or denitrified; however, little progress has been made in determining the relative importance of Nr accumulation and denitrificationDenitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
, which has been mainly due to a lack of integration among scientific disciplines.
Most Nr applied to global agroecosystem
Agroecosystem
An agroecosystem is the basic unit of study for an agroecologist, and is somewhat arbitrarily defined as a spatially and functionally coherent unit of agricultural activity, and includes the living and nonliving components involved in that unit as well as their interactions.An agroecosystem can be...
s cascades through the atmosphere and aquatic and terrestrial ecosystems until it is converted to N2, primarily through denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
. Although terrestrial denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
produces gaseous intermediates (nitric oxide [NO] and nitrous oxide [N2O]), the last step—microbial production of N2—is critical because atmospheric N2 is a sink for Nr. Many studies have clearly demonstrated that managed buffer strips and wetlands can remove significant amounts of nitrate (NO3-) from agricultural systems through denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
. Such management may help attenuate the undesirable cascading effects and eliminate environmental Nr accumulation.
Human activities dominate the global and most regional N cycles. N inputs have shown negative consequences for both nutrient cycling and native species diversity in terrestrial and aquatic systems. In fact, due to long-term impacts on food webs, Nr inputs are widely considered the most critical pollution problem in marine systems. In both terrestrial and aquatic ecosystems, responses to N enrichment vary; however, a general re-occurring theme is the importance of thresholds (e.g., nitrogen saturation
Saturation
Saturation or saturated may refer to:- Meteorology :* Dew point, which is a temperature that occurs when atmospheric humidity reaches 100% and the air is saturated with moisture- Physics :...
) in system nutrient retention capacity. In order to control the N cascade, there must be integration of scientific disciplines and further work on Nr storage and denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
rates. this is part of the nitrogen cycle
Further reading
- Good A. G. & Beatty P. H. (2011). "Fertilizing Nature: A Tragedy of Excess in the Commons". PLoS BiologyPLoS BiologyPLoS Biology is a peer-reviewed scientific journal covering all aspects of biology. Publication began on October 13, 2003.It was the first journal of the Public Library of Science. All content in PLoS Biology is published under the Creative Commons "by-attribution" license...
9(8): e1001124. doi:10.1371/journal.pbio.1001124. - Scarsbrook M.; Barquin J.; Gray D. (2007) New Zealand coldwater springs and their biodiversity. Science for Conservation 278. p 72. Department of Conservation, New Zealand
- Venterink, H. O., M. J. Wassen, A. W. M. Verkroost, and P. C. de Ruiter. 2003. Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 84:2191-2199

