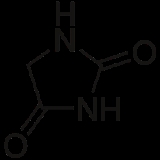
Hydantoin
Encyclopedia
Hydantoin, which is also known as glycolylurea, is a heterocyclic organic compound
that can be thought of as a cyclic "double-condensation reaction
" product of glycolic acid
and urea
. Its chemical structure
, shown in the Table of Properties at right, is similar to that of imidazolidine
except that the molecule
of hydantoin has carbonyl groups in the number 2 and 4 positions in the ring. Imidazolidine is the hydrogen
-saturated analogue of imidazole
. Imidazole is a heterocyclic aromatic organic compound.
In a more general sense, hydantoins can refer to chemical compound
s that have substituent groups
bonded to a hydantoin ring skeletal structure. For example, phenytoin
(mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.
in the course of his study of uric acid
. He obtained it by hydrogenation
of Allantoin
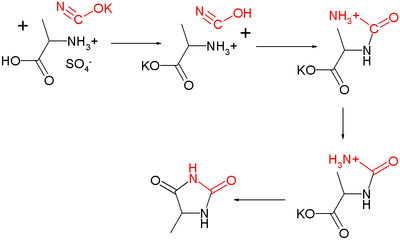
, hence the name. Urech in 1873 synthesized the derivative 5-methylhydantoin from alanine
sulfate
and potassium cyanate
in what is now known as the Urech hydantoin synthesis
:
 The 5,5-dimethyl compound can also be obtained from acetone cyanohydrin
The 5,5-dimethyl compound can also be obtained from acetone cyanohydrin
(also discovered by Urech: see cyanohydrin reaction
) and ammonium carbonate
. This reaction type is called the Bucherer–Bergs reaction.
According to the 1911 Encyclopedia Britannica, hydantoin can also be synthesized either by heating allantoin
with hydroiodic acid or by "heating bromacetyl urea with alcoholic ammonia".
, glycine
is one of the products.
is used in malignant hyperthermia
, neuroleptic malignant syndrome
, spasticity
, and Ecstasy intoxication.
Some N-Halogenated derivatives of hydantoin are used as chlorinating or brominating agents in disinfectant/sanitizer or biocide
products. The three major N-halogenated derivatives are dichlorodimethylhydantoin (DCDMH), bromochlorodimethylhydantoin (BCDMH
), and dibromodimethylhydantoin (DBDMH
).
and thymine
bases in DNA
are oxidized to hydantoins over time after the death of an organism. Such modifications block DNA polymerases and thus prevents PCR from working. Such damage is a problem when dealing with ancient DNA samples.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
that can be thought of as a cyclic "double-condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
" product of glycolic acid
Glycolic acid
Glycolic acid is the smallest α-hydroxy acid . This colorless, odorless, and hygroscopic crystalline solid is highly soluble in water. It is used in various skin-care products. Glycolic acid is found in some sugar-crops...
and urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
. Its chemical structure
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
, shown in the Table of Properties at right, is similar to that of imidazolidine
Imidazolidine
Imidazolidine is a heterocyclic compound formally derived by the addition of four hydrogen atoms to imidazole. The intermediate, resulting from the addition of only two hydrogen atoms is called dihydroimidazole...
except that the molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
of hydantoin has carbonyl groups in the number 2 and 4 positions in the ring. Imidazolidine is the hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
-saturated analogue of imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
. Imidazole is a heterocyclic aromatic organic compound.
In a more general sense, hydantoins can refer to chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s that have substituent groups
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
bonded to a hydantoin ring skeletal structure. For example, phenytoin
Phenytoin
Phenytoin sodium is a commonly used antiepileptic. Phenytoin acts to suppress the abnormal brain activity seen in seizure by reducing electrical conductance among brain cells by stabilizing the inactive state of voltage-gated sodium channels...
(mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.
Synthesis
Hydantoin was first isolated in 1861 by Adolf von BaeyerAdolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer was a German chemist who synthesized indigo, and was the 1905 recipient of the Nobel Prize in Chemistry. Born in Berlin, he initially studied mathematics and physics at Berlin University before moving to Heidelberg to study chemistry with Robert Bunsen...
in the course of his study of uric acid
Uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates such as ammonium acid urate. Uric acid is created when the body breaks down purine nucleotides. High blood concentrations of uric acid...
. He obtained it by hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
of Allantoin
Allantoin
Allantoin is a chemical compound with formula C4H6N4O3. It is also called 5-ureidohydantoin or glyoxyldiureide. It is a diureide of glyoxylic acid....
, hence the name. Urech in 1873 synthesized the derivative 5-methylhydantoin from alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
and potassium cyanate
Potassium cyanate
Potassium cyanate is an inorganic compound with the formula KOCN . It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.-Structure and bonding:Cyanate is isoelectronic with...
in what is now known as the Urech hydantoin synthesis
Urech hydantoin synthesis
The Urech hydantoin synthesis is the chemical reaction of amino acids with potassium cyanate and hydrochloric acid to give hydantoins....
:

Cyanohydrin
A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
(also discovered by Urech: see cyanohydrin reaction
Cyanohydrin reaction
A Cyanohydrin reaction is an organic chemical reaction by an aldehyde or ketone with a cyanide anion or a nitrile to form a cyanohydrin. This nucleophilic addition is a reversible reaction but with aliphatic carbonyl compounds equilibrium is in favor of the reaction products. The cyanide source...
) and ammonium carbonate
Ammonium carbonate
Ammonium carbonate is a commercial salt with the chemical formula 2CO3. It is used when crushed as a smelling salt. It can be crushed when needed in order to revive someone who has fainted...
. This reaction type is called the Bucherer–Bergs reaction.
According to the 1911 Encyclopedia Britannica, hydantoin can also be synthesized either by heating allantoin
Allantoin
Allantoin is a chemical compound with formula C4H6N4O3. It is also called 5-ureidohydantoin or glyoxyldiureide. It is a diureide of glyoxylic acid....
with hydroiodic acid or by "heating bromacetyl urea with alcoholic ammonia".
Chemical
When hydantoin reacts with hot, dilute hydrochloric acidHydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
is one of the products.
Derivatives
DantroleneDantrolene
Dantrolene sodium is a muscle relaxant that acts by abolishing excitation-contraction coupling in muscle cells, probably by action on the ryanodine receptor. It is the only specific and effective treatment for malignant hyperthermia, a rare, life-threatening disorder triggered by general anesthesia...
is used in malignant hyperthermia
Malignant hyperthermia
Malignant hyperthermia or malignant hyperpyrexia is a rare life-threatening condition that is usually triggered by exposure to certain drugs used for general anesthesia; specifically, the volatile anesthetic agents and the neuromuscular blocking agent, succinylcholine...
, neuroleptic malignant syndrome
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is a life- threatening neurological disorder most often caused by an adverse reaction to neuroleptic or antipsychotic drugs...
, spasticity
Spasticity
Spasticity is a feature of altered skeletal muscle performance in muscle tone involving hypertonia, which is also referred to as an unusual "tightness" of muscles...
, and Ecstasy intoxication.
Some N-Halogenated derivatives of hydantoin are used as chlorinating or brominating agents in disinfectant/sanitizer or biocide
Biocide
A biocide is a chemical substance or microorganism which can deter, render harmless, or exert a controlling effect on any harmful organism by chemical or biological means. Biocides are commonly used in medicine, agriculture, forestry, and industry...
products. The three major N-halogenated derivatives are dichlorodimethylhydantoin (DCDMH), bromochlorodimethylhydantoin (BCDMH
BCDMH
1-Bromo-3-chloro-5,5-dimethylhydantoin is a chemical structurally related to hydantoin. It is a white crystalline compound with a slight bromine and acetone odor and is insoluble in water, but soluble in acetone....
), and dibromodimethylhydantoin (DBDMH
DBDMH
DBDMH is an organic compound derived from the heterocycle called dimethylhydantoin. This white crystalline compound with a slight bromine odor is widely used as a disinfectant used for drinking water purification, recreational water treatment, as a bleaching agent in pulp and paper mills, and for...
).
Pharmaceutical Industry
Hydantoin is used to synthesize the following anticonvulsants:- ethotoinEthotoinEthotoin is an anticonvulsant drug used in the treatment of epilepsy. It is a hydantoin, similar to phenytoin. Ethotoin lacks phenytoin's side effects of gingival hyperplasia and hirsutism, however it is less effective. This, combined with the need for frequent dosing has limited its usefulness...
- phenytoinPhenytoinPhenytoin sodium is a commonly used antiepileptic. Phenytoin acts to suppress the abnormal brain activity seen in seizure by reducing electrical conductance among brain cells by stabilizing the inactive state of voltage-gated sodium channels...
- mephenytoinMephenytoinMephenytoin is a hydantoin, used as an anticonvulsant. It was introduced approximately 10 years after phenytoin, in the late 1940s. The significant metabolite of mephenytoin is nirvanol , which was the first hydantoin...
- fosphenytoinFosphenytoinFosphenytoin is a water-soluble phenytoin prodrug used only in hospitals for the treatment of epileptic seizures....
DNA Oxidation to hydantoins after cell death
A high proportion of cytosineCytosine
Cytosine is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine . It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached . The nucleoside of cytosine is cytidine...
and thymine
Thymine
Thymine is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. As the name suggests, thymine may be derived by methylation of uracil at...
bases in DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
are oxidized to hydantoins over time after the death of an organism. Such modifications block DNA polymerases and thus prevents PCR from working. Such damage is a problem when dealing with ancient DNA samples.

