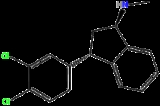
Indatraline
Encyclopedia
Indatraline is a non-selective
monoamine transporter
inhibitor that has been shown to block the reuptake
of dopamine
, norepinephrine
, and serotonin
with effects similar to those of cocaine
. However, the effects have been shown to have slower onset and longer duration than cocaine, suggesting that the compound may, along with similar compounds, be used for treatment of cocaine addiction. Apparently, Lu 19-005 can be used to block the action of methamphetamine
and MDMA.
Compare Indatraline with tametraline
since they are directly homologous. There are some differences though. Superposition
should make it possible to see that there is at least a relationship between the pharmacophore of indatraline and various phenyltropanes. More recently, additional work has been done also:
s though.
Measurement of ambulation was recording in 10-minute bins. Whereas for cocaine, LMA was already apparent at 10 min, at least 20 minutes were needed before the NMe2 analogs even started to increase locomotor activity. Very high doses actually (over the hill) meant less ambulation. The reason for this was increased stereotypy
if you overbake it. It is apparent from the table that larger N-groups result in increased DAT selectivity relative to the SERT and NET. Although it is not necessary to enter into the details, the authors actually wanted a SNDRI.
[125I
]RTI-55 used as the binding ligand at all 3 transporters.
Interestingly, the eudysmic ratio
between the two enantiomers of N-methyl-indatraline is not as high as was reported in the case of tametraline
. The toxicity of the R,S isomer is less than for the S,R isomer.
(Rac) DAT: 310, DA: 290. SERT: 17, 5-HT: 3700. NET: 1400, NE: 3200.
The idea is a method to gain increased DA(T) selectivity relative to the other transporters.
the other had been adapted to scale-up:
Although a new method of making indatraline was recently published, there is obviously more than one way of making this compound.
See for example: 6525206
Unfortunately for the indanone intermediates a method is not available for direct reduction of the imine
or oxime
. It is reported that the wrong diastereomers are formed (cis) whereas the trans isomers are the ones that are needed. This frustrates the synthesis since an extra step has to be inserted. First the ketones are reduced to get mostly cis alcohols, which are then converted to the corresponding mesylates conserving stereochemistry. These can then be reacted with e.g. N-methylbenzylamine, effecting a Walden inversion
(SN2). Final removal of the benzyl affords product, although it is racemic.
Binding selectivity
Binding selectivity refers to the differing affinities with which different ligands bind to a substrate forming a complex. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate...
monoamine transporter
Monoamine transporter
Monoamine transporters are protein structures that function as integral plasma membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. Three major classes of MATs are responsible for the reuptake of their associated amine neurotransmitters...
inhibitor that has been shown to block the reuptake
Reuptake
Reuptake, or re-uptake, is the reabsorption of a neurotransmitter by a neurotransmitter transporter of a pre-synaptic neuron after it has performed its function of transmitting a neural impulse....
of dopamine
Dopamine
Dopamine is a catecholamine neurotransmitter present in a wide variety of animals, including both vertebrates and invertebrates. In the brain, this substituted phenethylamine functions as a neurotransmitter, activating the five known types of dopamine receptors—D1, D2, D3, D4, and D5—and their...
, norepinephrine
Norepinephrine
Norepinephrine is the US name for noradrenaline , a catecholamine with multiple roles including as a hormone and a neurotransmitter...
, and serotonin
Serotonin
Serotonin or 5-hydroxytryptamine is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal tract, platelets, and in the central nervous system of animals including humans...
with effects similar to those of cocaine
Cocaine
Cocaine is a crystalline tropane alkaloid that is obtained from the leaves of the coca plant. The name comes from "coca" in addition to the alkaloid suffix -ine, forming cocaine. It is a stimulant of the central nervous system, an appetite suppressant, and a topical anesthetic...
. However, the effects have been shown to have slower onset and longer duration than cocaine, suggesting that the compound may, along with similar compounds, be used for treatment of cocaine addiction. Apparently, Lu 19-005 can be used to block the action of methamphetamine
Methamphetamine
Methamphetamine is a psychostimulant of the phenethylamine and amphetamine class of psychoactive drugs...
and MDMA.
Compare Indatraline with tametraline
Tametraline
Tametraline is the parent of a series of chemical compounds investigated at Pfizer that eventually led to the development of sertraline .Sertraline has been called "3,4-dichloro tametraline"...
since they are directly homologous. There are some differences though. Superposition
Superposition principle
In physics and systems theory, the superposition principle , also known as superposition property, states that, for all linear systems, the net response at a given place and time caused by two or more stimuli is the sum of the responses which would have been caused by each stimulus individually...
should make it possible to see that there is at least a relationship between the pharmacophore of indatraline and various phenyltropanes. More recently, additional work has been done also:
Trans
If indatraline is N-alkylated it is possible to slow the onset of action, if the choice of alkyl group is sufficiently bulky, so that it is not until N-demethylation occurs that the molecules become really active. N-methyl indatraline has a much longer duration than indatraline, because norindatraline is inactive whereas demethylating N-methyl-indatraline does not terminate the actions of the parent compound. Most drug addiction programs are of the opinion that a "slow onset, long duration" DRI is less likely to be abused (c.f. Volkow and others). The N-methyl products are clearly not just inactive prodrugProdrug
A prodrug is a pharmacological substance administered in an inactive form. Once administered, the prodrug is metabolised in vivo into an active metabolite, a process termed bioactivation. The rationale behind the use of a prodrug is generally for absorption, distribution, metabolism, and...
s though.
Measurement of ambulation was recording in 10-minute bins. Whereas for cocaine, LMA was already apparent at 10 min, at least 20 minutes were needed before the NMe2 analogs even started to increase locomotor activity. Very high doses actually (over the hill) meant less ambulation. The reason for this was increased stereotypy
Stereotypy
A stereotypy is a repetitive or ritualistic movement, posture, or utterance, found in people with mental retardation, autism spectrum disorders, tardive dyskinesia and stereotypic movement disorder. Stereotypies may be simple movements such as body rocking, or complex, such as self-caressing,...
if you overbake it. It is apparent from the table that larger N-groups result in increased DAT selectivity relative to the SERT and NET. Although it is not necessary to enter into the details, the authors actually wanted a SNDRI.
Racemic
N-Demethyl analogs situated on the right-side of the arrow, assuming that this is the way they are metabolized in vivo.[125I
Iodine-125
Iodine-125 is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat prostate cancer and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.Its half-life is around 59 days and it...
]RTI-55 used as the binding ligand at all 3 transporters.
| Racemic (trans) 3',4'-Dichloro Indamines. Ki and IC50 (nM) | ||||||||
| Stereo | R1 | R2 | DAT | DA | SERT | 5-HT | NET | NE |
| Rac | Me | Me | 19 → 27 | 200 → 23 | 0.33 → 5.0 | 65 → 4.8 | 95 → 22 | 34 → 15 |
| Rac | Et | Me | 39 → 8.1 | 270 → 61 | 16 → 2.0 | 24 → 13 | 250 → 48 | 150 → 28 |
| Rac | Pr | Me | 250 → 220 | 340 → 53 | 91 → 130 | 190 → 480 | 400 → 55 | 640 → 130 |
| Rac | Pri | Me | 180 → 32 | 190 → 51 | 44 → 93 | 1500 → 240 | 260 → 110 | 420 → 75 |
| Rac | But | Me | 890 → 130 | 2700 → 370 | 4700 → 740 | >10μM → 3100 | 440 → 150 | 1000 → 310 |
| Rac | Bn | Me | 120 → 80 | 550 → 380 | 180 → >10μM | 3700 → >10μM | 2800 → 460 | 1600 → 2600 |
Single Enantiomers
| Trans 3',4'-Dichloro Indamines. Ki and IC50 (nM) | ||||||||
| Stereo | R1 | R2 | DAT | DA | SERT | 5-HT | NET | NE |
| (±)-trans | Me | Me | 19 | 200 | 0.33 | 65 | 95 | 34 |
| (+)-trans | Me | Me | 8.7 | 120 | 0.06 | 6.3 | 52 | 21 |
| (–)-trans | Me | Me | 38 | 650 | 3.6 | 130 | 130 | 130 |
Interestingly, the eudysmic ratio
Eudysmic ratio
The Eudysmic ratio is a common term in pharmacology, chemistry, and molecular biology. The eudysmic ratio describes the difference in pharmacologic activity between the two enantiomers of a drug....
between the two enantiomers of N-methyl-indatraline is not as high as was reported in the case of tametraline
Tametraline
Tametraline is the parent of a series of chemical compounds investigated at Pfizer that eventually led to the development of sertraline .Sertraline has been called "3,4-dichloro tametraline"...
. The toxicity of the R,S isomer is less than for the S,R isomer.
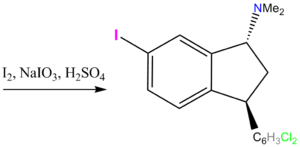
Iodination
This was actually done for radiological purposes, although the compound was tested anyway.
(Rac) DAT: 310, DA: 290. SERT: 17, 5-HT: 3700. NET: 1400, NE: 3200.
The idea is a method to gain increased DA(T) selectivity relative to the other transporters.
Cis
| Cis 3',4'-Dichloro Indamines. Ki and IC50 (nM) | |||||||
| R1 | R2 | DAT | DA | SERT | 5-HT | NET | NE |
| Me | Me | 63 → 290 | 550 → 500 | 2.4 → 38 | 5.5 → 4.0 | 150 → 600 | 86 → 130 |
| Et | Me | 660 → 140 | 530 → 1000 | 13 → 4.7 | 72 → 19 | 2400 → ? | 4400 → 170 |
| Pr | Me | 1200 → 280 | 1000 → 620 | 200 → 500 | 810 → 1300 | 2000 → 540 | 540 → 630 |
| Pri | Me | 680 → 450 | 960 → 1400 | 73 → 130 | 1800 → 300 | 650 → 3500 | 1800 → 1500 |
| But | Me | 2800 → 650 | >10μM → 1500 | >10μM → 1700 | ? → >10μM | 2200 → 1100 | >10μM → 1800 |
| Bn | Me | >10μM → 1100 | ? → 5300 | 300 → 2100 | 700 → >10μM | >10μM → 4800 | >10μM → >10μM |
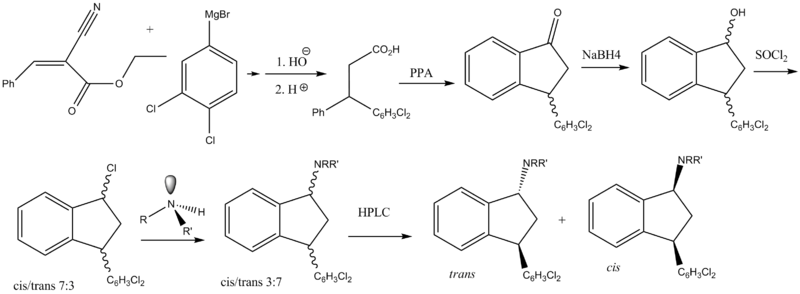
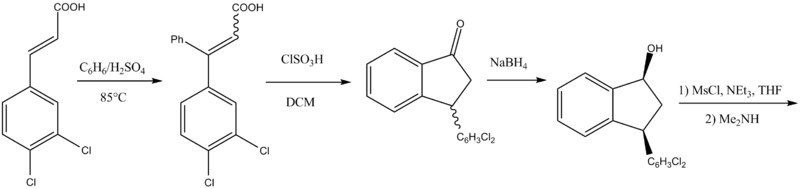
Chemistry
2 main routes have been reported. The first route shown is the original one reported by Bøgesø and co-workers.
the other had been adapted to scale-up:

Although a new method of making indatraline was recently published, there is obviously more than one way of making this compound.
See for example: 6525206
Unfortunately for the indanone intermediates a method is not available for direct reduction of the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
or oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
. It is reported that the wrong diastereomers are formed (cis) whereas the trans isomers are the ones that are needed. This frustrates the synthesis since an extra step has to be inserted. First the ketones are reduced to get mostly cis alcohols, which are then converted to the corresponding mesylates conserving stereochemistry. These can then be reacted with e.g. N-methylbenzylamine, effecting a Walden inversion
Walden inversion
Walden inversion is the inversion of a chiral center in a molecule in a chemical reaction. Since a molecule can form two enantiomers around a chiral center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in a SN2 reaction,...
(SN2). Final removal of the benzyl affords product, although it is racemic.

